Translation, transculturation and validity of the self-administered questionnaire for functionality (Systemic Sclerosis Questionnaires [SySQ]) for use in Spanish patients with systemic sclerosis and its relationship to the severity of the disease and to quality of life.
Patients and methodsWe conducted an observational analytical study to perform a cross-cultural validation of the self-administered questionnaire on functionality in scleroderma. The validity of the form and content was evaluated by an expert panel. The method included: (a) adaptation into Spanish of the construct for translation and back translation, and transculturation; (b) internal consistency with the SySQ (Cronbach's alpha), and (c) reproducibility was assessed taking into account all occasions in which the test was performed with Cohen's kappa. Additionally, we calculated the Spearman correlation coefficient with the Medsger severity scale, Health Assessment Questionnaire score and SF-36 score.
ResultsWe included 70 patients with systemic sclerosis: age 17–78 (51±12) years, 65 (93%) were women, diffuse/limited subtype 64/36%, disease duration of .5–40 years. Optimal internal consistency for all categories of the final version of SySQ (Cronbach's α of .961) and intraobserver reliability in 2 tests over a 2-week interval (Cohen's kappa coefficient .618) and optimal interobserver reliability in 2 tests on the same day (Cohen's kappa coefficient .911). Moderate correlation between functionality by SySQ and by Health Assessment Questionnaire (r=.573, P<.0001). Inverse correlation between SySQ and quality of life mental health domain SF-36 (r=−.435, P<.001) and physical domain SF-36 (r=−.638, P<.001). Medsger severity scale (tendon, heart, lung, vascular) also showed significant correlation with SySQ.
ConclusionsSySQ in this validated Spanish version is a suitable instrument to measure functional status in patients with systemic sclerosis. Reduced functionality is related to greater tendon and peripheral vascular involvement and to a poorer quality of life.
Traducción, transculturización y validez del cuestionario autoadministrado para funcionalidad (Systemic Sclerosis Questionnaire [SySQ]) en esclerosis sistémica al idioma español y su relación con la enfermedad y la calidad de vida.
Pacientes y métodosEstudio observacional analítico. Validación realizada por panel de expertos, en apariencia y contenido. La metodología incluyó: a) adaptación al español del constructo por traducción, retrotraducción, y transculturización; b) consistencia interna para todas y cada una de las categorías del SySQ (alfa de Cronbach), y c) la reproducibilidad se evaluó tomando en cuenta todas las ocasiones en que se realizó la prueba con kappa de Cohen. Adicionalmente calculamos el coeficiente de correlación de Spearman con: 1) escala de severidad de Medsger; 2) Health Assessment Questionnaire, y 3) prueba SF-36.
ResultadosSe incluyeron 70 pacientes con esclerosis sistémica, edad 17-78 (51±12) años, 65 (93%) mujeres, subtipo difuso/limitado 64/36%, evolución de la enfermedad 0,5-40 años. Observamos consistencia interna óptima de la versión final del SySQ (alfa de Cronbach 0,961) y buena reproducibilidad intraobservador entre pruebas con intervalo de 2 semanas (kappa de Cohen 0,618) y óptima interobservador el mismo día (kappa de Cohen 0,911). Moderada correlación entre los cuestionarios de funcionalidad SySQ y de discapacidad de Health Assessment Questionnaire (r=0,573; p<0,0001). Correlación inversa entre SySQ y calidad de vida en dominio mental del SF-36 (r=−0,435; p<0,001) y en dominio físico del SF-36 (r=−0,680; p<0,001). La escala de severidad de la enfermedad de Medsger (tendón, corazón, pulmón, vascular) también mostró correlación significativa con SySQ.
ConclusionesEsta versión en español del cuestionario autoadministrado SySQ es un instrumento válido para evaluar el estado funcional de pacientes con esclerosis sistémica. La menor funcionalidad está relacionada con una mayor afección a tendón y vascular periférico y con una menor calidad de vida.
Systemic sclerosis (SS) or scleroderma is a rheumatologic disorder which mainly affects women, leading to vasculopathy, autoimmunity and fibrosis of the skin and visceral organs.1 During its initial stage, progressive, vascular dysfunction predominates and involves organs such as the skin, lung, heart, muscles, tendons, digestive tract, kidney and general health status.2 Many methods have been proposed for the measuring of SS activity, prognosis and assessment of therapeutic response, but few assess functionality. The speed of disease progression is highly varied for each individual and functionality is the main indicator of systemic improvement or impairment. The disability index of the Health Assessment Questionnaire (HAQ) is a tool which predicts survival. Although the HAQ is used in SS, it was designed for patients with rheumatoid arthritis. Its adaptation to SS (scleroderma Health Assessment Questionnaire) bears little correlation to disease severity.3 In 1998, a functionality scale was designed in England, but the patients qualified their disability as considerably higher than their therapist and it was not used.4 Another test refers solely to hand mobility in sclerodermia or the HAMIS Test.5 In view of this situation Ruof et al.6 designed a self-administered functionality questionnaire for SS (Systemic Sclerosis Questionnaire [SySQ]), which assesses the difficulty in carrying out everyday life activities and the intensity and frequency of symptoms with the highest functional impact in SS. This instrument was originally designed in German and has been validated into other languages, but not into Spanish. Scales were recently published for SS assessment but no functionality scale was specifically included for SS,7 and it was therefore our objective to validate the SySQ functionality scale transculturally in Mexican patients with SS and assess its relationship with the HAQ disability scale, disease severity and quality of life.
Patients and MethodsWe conducted an observational analytical study in the Internal Medicine Department of the Specialities Hospital of the La Raza Medical Centre, of the Mexican Social Security Institute. We included all patients of a cohort diagnosed with SS, according to the criteria of the American College of Rheumatology of 1980 and confirmed in their classification with the American College of Rheumatology and the European League Against Rheumatism of 2013,8 who knew how to read and write and who signed the consent form. Excluded patients were those with syndromes overlapping clinical evaluation, mixed connective tissue disease, comorbidities affecting quality of life which were different from SS or those with impediments to questionnaire response. The protocol was approved by the local research and research ethics committee, registered with number R-2010-3501-40.
Phase 1. Translation and TransculturationTranslation and transculturation followed methodology used by other studies recommended by Falcão et al.9 The tool was initially translated from German into Spanish by a native translator, creating the first Spanish version. The initial translation was reverted into the original language and later compared with the original tool. Any discrepancies found were jointly reviewed by an internist, a rheumatologist and the translator, adapting them and generating a second Spanish version. The semantic equivalence was analysed based on the vocabulary and corresponding grammar, idiomatic equivalence, translation of idiomatic expressions and conceptual equivalence, since the terms could be semantically equivalent but not conceptually so.
Following translation of the SySQ questionnaire a content assessment was made by a panel of 5 experts (2 rheumatologists, 2 clinical researchers, one internist). These included 3 domains concerning daily life activities, intensity and frequency of symptoms. Three domains were distributed into 4 categories: (1) general symptoms; (2) musculoskeletal symptoms; (3) cardiopulmonary symptoms, and (4) digestive tract symptoms, which are the main organs affected and represent the highest impact on functionality of patients with SS. This scale includes a section on the capacity to take an action (0=no difficulty, 1=a little difficulty, 2=some difficulty, 3=unable to do); another section describes the intensity of symptoms (0=none, 1=mild, 2=moderate, 3=very intense), and finally there is a section on symptom frequency (0=no, 1=sometimes, 2= often, 3=always). The final score of the scale is calculated by averaging the maximum value obtained in each domain (block of questions) divided between 3 (given that there are 3 domains), and this value ranges from 0 (normal functionality) to 3 (minimum functionality).
This version was later applied to a group of 10 patients, adding to each of the questions the option “not applicable” so as to identify the questions which were not understood or were not used regularly by our population and therefore were considered as culturally inappropriate. Questions with over 25% of “not applicable” were analysed by the committee and then replaced with others with the same concept. Patient opinions on the clarity and facility to respond to reactions were considered and appearance was corrected on 2 occasions and the content in 3 questions. These changes led to the new version which was re-applied to the patients, until “not applicable” was not obtained in over 15% of patients, generating the Mexican version to the Spanish version of the questionnaire.
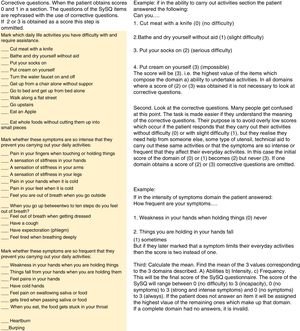
Phase 2. Application of the Questionnaire and Assessment of the Psychometric MeasurementsIn phase 2 we included 70 consecutive patients in follow-up visits out of a cohort of 210 patients with SS. The SySQ functionality self-administered questionnaire (Fig. 1) was administered, and adjusted with corrective questions which are shown and explained in Fig. 2. The disability self-questionnaire (HAQ) was applied, as was SF-36 quality of life quesionnaire,10 and the Medsger11 severity index. The latter took into account laboratory and imaging studies which had been taken no more than 3 months after the appointment date.
Mexican version in Spanish of the self-administered functionality scale of the Systemic Sclerosis Questionnaire.
Score. First. 32 scores were obtained in 3 domains. We chose the highest score for each domain (11 responses to ability, 12 to intensity and 9 to frequency). Initial scores were therefore reduced to 3. Second. To avoid confusions in low scores (0 and 1) a requirement was made to look at the corrective questions, as described in the section on Material and method. Third. The mean of the 3 values of the domains was calculated: (a) abilities, (b) intensity, and (c) frequency. This will be the final score of the SySQ questionnaire. Interpretation: 0=no difficulty or no symptoms; 1=slight difficulty, presences of mild symptoms and/or on some occasions; 2=serious difficulty, moderate symptoms and/or symptoms present frequently, and 3=incapacity, strong or intense symptoms which always present.
The final version of the translated questionnaire was assessed by 3 interviews with each patient: initially by 2 different interviewers to assess interobserver reproducibility and 14 days later by the first interviewer to assess intraoberver reproducibility. The SySQ questionnaire responses from the first interview were taken for internal consistency analysis using Cronbach's alpha. Reproducibility was assessed by taking into account all the occasions when the Cohen kappa test was performed. To relate the SySQ self-administered questionnaire with scales which could affect functionality, we calculated Spearman's coefficient with the HAQ disability scale and with Medsger's disease severity scale as a good indicator of specific damage to organs.12 We used the SF-36 quality of life questionnaire proposed as the appropriate one for this variable related to health in sclerodermia in a meta-analysis.13,14
Clinical and Paraclinical Data CollectionThe clinical examination was performed the same day as the functional assessment for each patient included in the study. The paraclinical parameters were accepted within an interval of the last 3 months, routinely collected during follow-up. We included age, gender, SS subtype (diffuse or limited), duration of the disease from the first SS symptom and the antibody associated with sclerodermia by immunoenzymatic analysis. The severity of the skin and internal organ involvement was assessed using a scale modified by Rodnan and the Medsger scale, respectively.11,15 Peripheral vascular involvements was assessed with the Medsger in gangrene or amputation, ulcers and scars. In the absence of these data the spontaneous active Raynaud phenomenon was assessed or placing the hands in water at 12°C for 2–5min to search for a change in colouring to paleness, hyperaemia or cianosis.16
Statistical AnalysisWe calculated the sample size as 47 patients by considering the minimum correlation of .4, plus 10% of possible losses and with a statistical power of 80% we re-calculated the sample as 52 patients. Initially the simple was random but many people did not meet with the inclusion criteria and subjects were consecutively included from the whole cohort.
The results with normal distribution are presented as a mean±standard deviation. Ordinal variables are expressed as percentage, median and minimum and maximum range.
We used Chronbach's alpha to assess the internal consistency of the questionnaire in its final version applied in the first interview, the kappa coefficient, to identify the proportion of concordance observed between the first and second response to the questionnaire in one same day (interobserver) and between the initial and another 2 weeks later (intraobserver), taking as values between 0 (no concordance) and 1 (maximum concordance).
The SySQ self-administered questionnaire was considered valid if a Crohbach alpha of >.8 was obtained and a Cohen kappa of >.6. We identified adequate or good correlation with the HAQ disability scales, quality of life (SF-36) and Medsger disease severity.7,10–14 Statistical analysis was performed with SPSS v21 software.
ResultsWe included 70 patients with SS, of 51.32±11.95 (17–78) years of age, 65 (93%) women, 45 (64.3%) of whom presented with the diffuse cutaneous variety, and 25 (35.7%) with limited cutaneous type, 5(7%) of them without dermal involvement or “sine sclerodermia”.17,18 Their evolution was 7 (.5–40) years (Table 1). The organs most frequently involved were skin (93%), vascular (77%) and gastrointestinal (77%) system; the least affected was the kidney (8.5%) (Table 1).
Patient Characteristics.
| Demographics | ||
|---|---|---|
| Age (years), mean±standard deviation | 51±12 | |
| Gender, n (%) | ||
| Female | 65 (93) | |
| Male | 5 (7) | |
| Evolution (years), median (min-max) | 7 (.−40) | |
| Clinical presentation. Subtype of systemic sclerodermia, n (%) | ||
| Diffuse cutaneous | 45 (64) | |
| Limited cutaneous | 25 (36) | |
| Antibodies, n (%)a | ||
| Antinuclear antibodies | 67 (88.2) | |
| Nucleolar | 24 (35.3) | |
| Anti-Scl70 | 16 (23.5) | |
| Anticentromere | 14 (20.6) | |
| Anti-RNP | 7 (10.3) | |
| Organ or system | Median (min-max) | Affected patients, n (%) |
| General state of health | 0 (0–3) | 18 (26) |
| Peripheral vascular | 1 (0–4) | 54 (77) |
| Skin | 1 (0–4) | 65 (93) |
| Tendon | 1 (0–4) | 41 (59) |
| Muscular | 1 (0–3) | 46 (66) |
| Gastrointestinal tract | 1 (0–2) | 54 (77) |
| Lung | 1 (0–4) | 38 (54) |
| Heart | 0 (0–4) | 15 (21) |
| Kidney | 0 (0–2) | 6 (9) |
Anti-RNP: anti-ribonuceloprotein antibodies; max: maximum; min: minimum.
Each of the 3 domains of SySQ were affected to some extent in around 70% of cases. Functionality (SySQ) was most frequently affected in the capacity for pressure or in reaching (82.9%) and less regularly when walking (57.1%); the total score was 1.66 (.6–3.0) (Table 2). Frequency of symptoms was high in 10% of patients and in 58.5% to a lesser extent, and for this reason was the domain with the highest score (2.1–3). Although ability to undertake daily life activities affected 70% of the patients, this was to a very low degree, and obtained the lowest score (1.0–3) (Table 2). The internal consistency was as good as the original tool, since Cronbach's alpha was .96 for the total of the tool and in the original article Cronbach's alpha was .93. We observed good reproducibility of the baseline test in duplicate on the first day and assessed by different researchers (Cohen kappa .911) and a second test with a 2-week interval by the same researcher (Cohen kappa of .618).
Seventy percent (70%) of the patients included manifested affected functionality, mainly classified as “with some difficulty”, and the symptoms which limited their functionality were mostly “of moderate intensity” and “often” in frequency (Table 2).
The HAQ disability questionnaire uses a total score range from 0 to 3, similar to SySQ. With HAQ we obtained a total score of a little less than that obtained with SySQ (1.0–2.8). The 8 domains of the HAQ scale were affected in 58%–65% of the patients, and with a mild disability median (Table 3). The abilities domain of the SySQ scale also had a median in the range described as slight difficulty on carrying out everyday activities (Tables 2 and 3). Spearman's correlation coefficient between the HAQ and SySQ scales was moderate (r=.573, P=.0001).
Health Assessment Questionnaire Disability Scale.
| Domain | Median (min–max) | Affected patients, n (%) |
|---|---|---|
| Get dressed | 1 (0–3) | 41 (58) |
| Get up | 1 (0–3) | 41 (58) |
| Eat | 1 (0–3) | 45 (64) |
| Walk | 1 (0–3) | 43 (62) |
| Hygiene | 1 (0–3) | 39 (56) |
| reach | 1 (0–3) | 45 (64) |
| Pressure | 1 (0–3) | 45 (64) |
| Others | 1 (0–3) | 46 (65) |
max: maximum; min minimum.
We use the SF-36 (best 0-worst 100) questionnaire to analyse quality of life. In the physical domain we found 35.9 (2.8–82.5). The domain which most affected patients was body pain (98.4%; 45.0–100), followed by physical role (45.7%; .0–100) (Table 4). The mental domain affected 45% (9.3–90.8), vitality and mental health 100% of patients (50.10–100 and 48.10–96, respectively), and the emotional role affected 75.7% (3.3, 0–100) (Table 4).
SF-36 Quality of Life Questionnaire.
| Domain | Median (min-max) | Cronbach's alpha |
|---|---|---|
| Physical control | ||
| Physical function | 47.5 (0–100) | .938 |
| Physical role | 45.7 (0–100) | .874 |
| Body pain | 45 (0–100) | .944 |
| General health status | 27.5 (0–85) | .867 |
| Mental control | ||
| Emotional role | 33.3 (0–100) | .662 |
| Social function | 50 (0–100) | .87 |
| Vitality | 50 (10–100) | .878 |
| Mental health | 48 (10–96) | .806 |
max: maximum; min minimum.
The Spearman correlation was inverse between HAQ and the mental domain of the SF-36 (r=−.390, P=.001) and the physical domain of the SF-36 (r=−.620, P=.0001). The relationship was also inverse between the functionality SySQ scale and the mental domain of the SF-36 (r=−.435, P=.0001) and the physical domain of the SF-36 (r=−.680, P=.0001) (Table 5)
Spearman's Correlation of Systemic Sclerosis Questionnaire With Health Assessment Questionnaire, SF-36 and Medsger.
| Correlation coefficient | P | |
|---|---|---|
| HAQ | .573a | <.001 |
| SF-36 | −.564a | <.001 |
| Mental control | −.435a | <.001 |
| Physical control | −.638a | <.001 |
| Medsger | .563 | <.0001 |
| General | −.044 | .716 |
| Peripheral vascular | .401a | .003 |
| Skin | .202 | .093 |
| Tendon | .458a | <.0001 |
| Muscular | .260b | .029 |
| Gastrointestinal | .194 | .107 |
| Pulmonary | .360b | .002 |
| Cardiac | .336b | .004 |
| Renal | .116 | .339 |
HAQ: Health Assessment Questionnaire.
The SySQ correlation was moderate with the severity of the Medsger disease in the tendon (r=.458; P<.0001), and adequate for the lung, heart, vascular and muscular system (Table 5).
The degree of limitation of everyday life SySQ activities with the overall Medsger severity scale had a moderate to good correlation (r=.563, P<.0001), which shows that the degree of dysfunction in everyday activities has a direct correlation with the degree of involvement to organs in SS. Analysis by domains with the best correlation was the tendon and the intensity of the Raynaud phenomenon (r=.401, P<.003) (Table 5).
DiscussionThe Spanish version of the functionality questionnaire of systemic sclerosis (SySQ) proved to be valid and reliable for Mexican patients with SS, using a methodology for transition and transculturation which is reproducible and reflects to some extent the severity of organ involvement.
In contrast to other rheumatic diseases, few questionnaires have been designed for patients with SS: As a result, functional limitation in these patients has been measured in clinical trials by the HAQ-DI disability index, a tool developed to measure disability in patients with rheumatoid arthritis. Other tools have been developed and validated, the majority focusing on specific organs involved in the SS.10,11 This tool was previously published by our group as a summary presented in a congress,19 and later translated and validated into Portuguese and Italian.20,21 In Mexico there are no specific tools in the Spanish language to assess functionality in these patients.
Ruof et al.6 developed the questionnaire in German, covering general symptoms, in addition to gastrointestinal, cardiopulmonary and musculoskeletal ones. We assessed the SySQ, HAQ and SF-36 scales in order to find the differences and similarities on classifying the ability of patients to undertake everyday activities and the relationship with the damage to different organs. We found there was a good relationship between functionality and the severity of the Raynaud phenomenon, because as this is one of the initial signs of the disease it represents an impact from the beginning of SS. Also with tendon involvement, which usually appears during the progressive course of the disease. Other domains which were statistically significant in the relationship between functional status and severity of the disease were muscular, cardiac and pulmonary signs as a direct link between functionality and organicity assessable through the SySQ questionnaire.
In our study, the questionnaire translated into Spanish proved to be reproducible, and usable in the Mexican population. The validity and transcultural adaptation of the original tool was achieved for application to the Mexican population.
In our study we found a similar spectrum of patients to those found in previous studies where functionality indexes for SS were tried, although the tools used were not specific to this disease.5–7
The importance of our study is based on the objective data provided by the SS clinic of our hospital for a comprehensive assessment of the patients and this afforded us the necessary elements for correct application of the severity indexes.
It is important to consider that the questionnaire does not directly assess the Raynaud phenomenon or renal involvement but the presence of pain is an indirect detail demonstrating the existence of this vascular phenomenon and renal involvements is infrequent in the Mexican population.22 Furthermore, the psychological sphere of the patient has an effect on the self-appreciation of their abilities to carry out daily life, frequency and intensity of symptoms, with consequent greater or lesser appreciation of the same. The psychological area is not considered in the SySQ, and could therefore be complemented with other tools which are able to identify this concern.
ConclusionsThe Spanish version of the SySQ questionnaire is valid, reliable, reproducible and accurate for the assessment of functionality in the Mexican population with SS. Our Spanish version of the self-administered SySQ functionality questionnaire had a coherent and inverse relationship with the HAQ disability scale, and with the physical and mental sections of the SF-36 quality of life scale. It also proved to be more closely related to the severity of the involvement of the tendon and peripheral vascular system in SS: The construction of new and better tools are required to allow us to assess not just the severity of symptoms but also the activity and chronicity of SS. The assessment of functionality in SS many also be an indirect indicator of treatment response.
Ethical LiabilitiesProtection of people and animalsThe authors declare that the procedures followed complied with ethical guidelines of the committee responsible for human experimentation and comply with the World Medical Association and the Declaration of Helsinki.
Data confidentialityThe authors declare that they have adhered to their centre of work on the publication of patient data.
Right to privacy and informed consentThe authors declare that they have obtained the informed consent from the patients and/or subjects referred to in this article. This document is held by the corresponding author.
Conflict of InterestsThe authors have no conflicts of interests to declare
The authors would like to thank the teacher Hannelore Rayle Staib for translation of the SySQ scale from German into Spanish.
Please cite this article as: Cruz-Domínguez MP, Casarrubias-Ramírez M, Gasca-Martínez V, Maldonado García C, Carranza-Muleiro RA, Medina G, et al. Cuestionario de funcionalidad para esclerosis sistémica (SySQ): validación en español del original en alemán y su relación con la enfermedad y la calidad de vida. Reumatol Clín. 2019;15:282–288.