Since their introduction almost 2 decades ago, biologic drugs have proven to be highly effective tools for the treatment of patients with inflammatory chronic rheumatic diseases1 which respond poorly to conventional disease-modifying antirheumatic drugs (DMARDS).2 Despite their ability to improve the quality of life of many of these patients, the high cost of these drugs, which is due to the complexity of their development and production,3 has limited their commonplace usage.4 It is expected that the drop in prices stimulated by competition from the arrival of biosimilar drugs will encourage the use of biologics in earlier stages of diseases, on the one hand, and on the other, will lead to greater equity in their availability.5 Although we are unaware of specific cases, we frankly do not believe there is any general inequity of biologic drug availability in the Spanish health system.
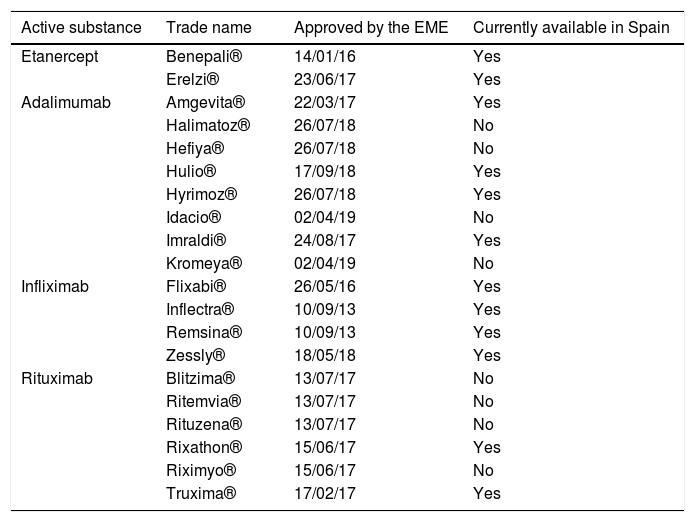
A biosimilar is a biologic drug which contains a version of the active substance of an original biologic agent that has already been approved (benchmark product), and which may be used in the same way, and through extrapolation, under the same indications as the product it imitates. For their approval, biosimilars were subjected to “head to head” clinical trials against their benchmark product, where they had to demonstrate that their safety and efficacy profile was indistinguishable from that of the drug they were compared with. Nowadays in Spain biosimilars of infliximab, etanercept, rituximab and adalimumab are available and it is highly likely that new biosimilars of these and other biologics prescribed for rheumatic diseases will be available in the near future. Table 1 shows the biosimilars of these molecules which have been approved up until now by the European Medicines Agency (EMA) and which ones are available in Spain.
Dates of approval by the EME of biosimilar drugs prescribed in rheumatology and their availability in Spain.
| Active substance | Trade name | Approved by the EME | Currently available in Spain |
|---|---|---|---|
| Etanercept | Benepali® | 14/01/16 | Yes |
| Erelzi® | 23/06/17 | Yes | |
| Adalimumab | Amgevita® | 22/03/17 | Yes |
| Halimatoz® | 26/07/18 | No | |
| Hefiya® | 26/07/18 | No | |
| Hulio® | 17/09/18 | Yes | |
| Hyrimoz® | 26/07/18 | Yes | |
| Idacio® | 02/04/19 | No | |
| Imraldi® | 24/08/17 | Yes | |
| Kromeya® | 02/04/19 | No | |
| Infliximab | Flixabi® | 26/05/16 | Yes |
| Inflectra® | 10/09/13 | Yes | |
| Remsina® | 10/09/13 | Yes | |
| Zessly® | 18/05/18 | Yes | |
| Rituximab | Blitzima® | 13/07/17 | No |
| Ritemvia® | 13/07/17 | No | |
| Rituzena® | 13/07/17 | No | |
| Rixathon® | 15/06/17 | Yes | |
| Riximyo® | 15/06/17 | No | |
| Truxima® | 17/02/17 | Yes |
EMA: European Medicines Agency.
For some time, the biosimilar market has been under pressure to reduce prices. This trend began in Northern countries and has extended to the United Kingdom, France, Germany, Italy and Spain. This has led manufacturers of biosimilars to offer discounts of up to 70% compared with the originator agent, although with significant variations both between countries and within the same country. This policy has pushed companies producing originator agents to reduce prices in order to retain their market, leading to a highly significant drop in the cost per patient of biologic drugs that have biosimilars. Furthermore, the Spanish Society of Rheumatology (SSR) has stated its unequivocal alignment with the sustainability of the healthcare systems and has shown its preference for the use of therapies at a lower cost when they have been proven to be comparable in efficacy and safety to the more expensive alternatives.6
The arrival of many biosimilars of products with which rheumatologists are familiarized and with unimaginable prices from only just 6 months ago will impact the prescription habits of biologics in rheumatology. A new era is already upon us and will present new challenges for the rheumatologists:
- 1.
The arrival of all these new molecules fortifies the need to prescribe biologics by their trade name in order to ensure their traceability. Different countries have adopted different approaches to guarantee accurate traceability of biologic products. The EU approved legislation which enforces the recording of the trade name and lot number, 7 whilst U.S.A. and Japan have continued to use specific non registered names. This fact has resulted in the name of biosimilars becoming complex and inconsistent on a worldwide level. It is essential that all biologic products can be identified with a single name. This applies to both biosimilars and the original products and is particularly important for prescription, traceability of the product and pharmacovigilance. As a result, the prescription of biologics including biosimilars must always be made with the trade name.
- 2.
As several biologics exist with biosimilars and also several biosimilars exist with the same benchmark molecule, this increases the complexity of names to be remembered, which may lead to the possibility of error.
- 3.
As we have already commented upon, during the last few months in many autonomous communities in Spain highly aggressive price policies have appeared where several biosimilars have been on offer at a price 4 times lower than the benchmark product, positioning the DMARDS price range to that of subcutaneous methotrexate. This is in itself good news, and will undoubtedly impact current prescription habits of rheumatologists today. On the one hand there will logically be a prioritization of the prescription of biosimilars in patients who are biologic naïve, compared to the prescription of their original innovators and also to other molecules with different targets, be they biologics or not, which are accepted as first line treatment. On the other hand, this price reduction policy will increase the pressure by health managers on rheumatologists to exchange originator biological agents with biosimilars in patients with a good clinical response. In this respect the prevailing law in Spain impedes the replacement by the hospital pharmacists of biologic products and the Spanish Society of Rheumatology (SSR), in its latest positioning on biosimilars6 defends that the exchange of a biologic for its biosimilar may only be made by the prescribing physician and that this exchange is accepted only in the case of patients in a stable condition. In any event, the SSR states that this should be an individualized decision made with the patient's consent.
- 4.
Current commercial aggressiveness may lead hospital pharmacies to change to biosimilars of the same molecule over time, and may lead to hospital managers recommending exchange between biosimilars with the same originator agent. These situations should be carefully analyzed since: (1) there are no studies to endorse the safety and effectiveness of exchange between biosimilars; (2) this complicates product traceability; (3) it encourages a market-based vision of medicine; and (4) it supports the concept that biosimilars are genetic products, which is not the case.
Substantial cost savings may undoubtedly be made if patients who are “naive” to biologics begin treatment with biosimilars. In patients who are already being treated with biologics and who show good response to them, changing to a biosimilar may be another source of saving, but any harmful effect in these circumstances must be taken into account. This effect may be responsible for a subjective increase in disease activity which would lead to lack of adherence. We believe optimum conditions for changing to biosimilars in patients with a biological treatment include: remission or low activity, rapid response (under 6 months) and as first or second line treatment.
In order to guarantee optimum and rational integration of biosimilars within rheumatology practice and to take advantage of their cost-saving opportunities, rheumatologists have to be aware that truthful information to our patients on cost-saving, efficacy and safety of biosimilars is the key to ensuring their usage long term. We understand that the course of action with the highest impact for the rational use of biosimilars is recognition by doctors, patients and healthcare authorities of the need to generate savings for the sustainment of our health system. Only from dialogue based on knowledge and comprehension towards patient sensitivity can rational and consensual usage of biologics and biosimilars be made in this new upcoming era.
FinancingNo financing was received for this study.
Please cite this article as: Díaz-González F, Bustabad-Reyes S. Los biosimilares, una nueva era en la reumatología en España. Reumatol Clín. 2020;16:131–132.