To describe the clinical–biological characteristics of patients with scleroderma (SS) and pulmonary artery hypertension (PAH).
To establish the relationship between pulmonary functional tests (PFT), Doppler echocardiography (ECHO) and the severity of the PAH.
Material and methodsRetrospective study of patients with SS treated at a tertiary center. All participants received a protocol study, which included a complete analysis and additional tests: ECHO and PFT with carbon monoxide diffusing capacity (DLCO).
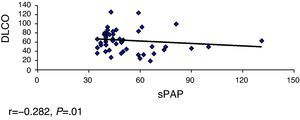
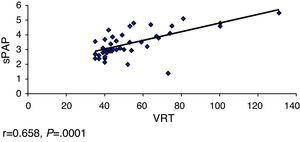
ResultsOverall, 331 patients were treated, including 68 (20.5%) with PAH. The limited subtype of SS was the most prevalent. The Pearson's correlation coefficient was used for the following variables: FVC-sPAP, FVC/DLCO-sPAP, DLCO-sPAP and TRV-sPAP, showed a significant moderate linear association in the relationship DLCO-sPAP and TRV-sPAP.
29 deaths occurred, with 12 of them related to PAH. The median time between the PAH diagnosis and death was 1.8 years.
ConclusionsThe decrease in DLCO and the increase in TRV are negative predictor factor of PAH which, at the same time, means a worsening prognosis for patients with SS.
Describir las características clínico-biológicas de pacientes con esclerodermia (ES) e hipertensión arterial pulmonar (HTAP).
Establecer la relación entre las pruebas funcionales respiratorias (PFR), la ecocardiografía Doppler (eco-Doppler) y la gravedad de la HTAP.
Material y métodosEstudio retrospectivo de pacientes con diagnóstico de ES seguidos en un centro de tercer nivel. Se les realizó un estudio protocolizado con analítica completa y pruebas complementarias; se estimó la presión arterial pulmonar sistólica (PAPs), la velocidad de reflujo de la válvula tricúspide (VRT), la difusión de monóxido de carbono (DLCO) y la capacidad vital forzada (CVF), por medio de la eco-Doppler y la PFR.
ResultadosSe incluyó a 331 pacientes, de los cuales 68 (20,5%) tenían HTAP. El subtipo de ES más prevalente fue la limitada. Se calculó el coeficiente de correlación de Pearson a las siguientes variables: CVF-PAPs CVF/DLCO-PAPs, DLCO-PAP y VRT-PAPs, observándose una asociación lineal moderada significativa en la relación DLCO-PAPs y con VRT-PAP.
Se constataron 29 fallecimientos, 12 relacionados a la HTAP. El tiempo medio entre el diagnóstico de HTAP y la muerte fue de 1,8 años.
ConclusionesLa disminución de la DLCO y el aumento de la VRT son factores predictores de HTAP, que al mismo tiempo condiciona un peor pronóstico en los pacientes con ES.
Pulmonary involvement in patients with SS is considered, after gastrointestinal complications, a frequent visceral complication, with a prevalence of approximately 80%. This complication may be due to diffuse involvement of the pulmonary interstitium or the appearance of pulmonary hypertension (PHT). The latter with a prevalence estimated at 12%–26% depending on diagnostic criteria, from scanning and population estudiada.1–3
PHT is generally considered a late complication of SS. Previous studies found that the TIME between the diagnosis of SS and the beginning of the PHT is between 9.08±6.64 and 14±5 years.1 It is also a major cause of morbidity and mortality in patients, with a median survival of 50% at 12 months of diagnosis.3,4
The definitive diagnosis of PHT is made with right heart catheterization; however, there are another 4 screening measurements used in most studies due to good correlation with the hemodynamic values obtained with cardiac catheterization: first, the measurement of pulmonary artery systolic pressure (sPAP); second, the speed of the tricuspid reflux (VRT), both parameters measured by transthoracic Doppler; third, considered as a predictor, the decrease of the diffusion coefficient of carbon monoxide (DLCO) calculated by means of the RFT, and finally the elevation in serum N-terminal brain natriuretic peptide (N-terminal BNP) prohormone, recently reviewed.5–7
In this paper, we studied a population with SS and PHT with clinical, immunologic, echocardiographic and spirometry parameters, and tried to identify predictors of higher levels of sPAP. We also studied the influence of PHT in the prognosis of these patients.
Materials and MethodsDesignThe study included 331 patients with a diagnosis of SS controlled at the Systemic Autoimmune Diseases Unit of the Internal Medicine department, University Hospital Vall d’Hebron, until 2009. Of these patients, we studied 68 patients who met PHT criteria by duplex Doppler considering PHT sPAP values above 35mmHg.
We divided the population according to clinical characteristics in subtypes of SS (limited, diffuse and no SS). These patients underwent spirometry with DLCO and FVC measurements, a computed tomography (CT), an immunological profile and capillaroscopy following a study protocol used in the department.8
Two patterns were considered for capillaroscopy: slow, characterized by the presence of megacapillaries, no hair loss, and a current pattern for important9 hair loss.
Statistical AnalysisStatistical analysis was performed using SPSS 11.5 for Windows. The association between variables was performed using the Pearson correlation coefficient and results were considered statistically significant if presenting P values of less than .05.
Results331 patients with SS were included in the study, of which 68 (20.5%) had sPAP>35mmHg. The mean age of patients was 56.3±14.4 years), 61 were women (90%) and 7 men (10%), with a 9:1 ratio.
Raynaud's phenomenon was the first most common manifestation of SS in all subtypes studied. The average time from the first manifestation of the disease until the diagnosis of the same was 9.5±11 years. Clinical and immunological characteristics of the study population are shown in Table 1.
Clinical, Immunological and Complementary Test Characteristics in Patients With SS and PTH.
| Diffuse | Limited | No Scleroderma | |
| Number of patients | 16/68 (24%) | 41/68 (60%) | 11/68 (16%) |
| Clinical characteristics | |||
| Raynaud's phenomenon | 12/16 (75%) | 38/41 (92%) | 10/11 (91%) |
| Capillaroscopy pattern | |||
| Slow | 10/16 (62%) | 36/41 (87%) | 9/11 (81%) |
| Active | 5/16 (32%) | 5/41 (13%) | 0 |
| Undefined | 1/16 (6%) | 0 | 2/11 (18%) |
| Presence of ulcers | 15/16 (93%) | 30/41 (73%) | 1/11 (9%) |
| Immunological | |||
| ACA (+) | 3/16 (19%) | 18/38 (47%) | 8/10 (72%) |
| Scl70 (+) | 9/16 (67%) | 3/38 (8%) | 1/11 (9%) |
| RFT | |||
| DLCO (media) | 55% (n=13) | 48% (n=29) | 53% (n=7) |
| CVF (media) | 51% (n=14) | 63% (n=36) | 61% (n=10) |
| TAC | |||
| Interstitial affection | 12/16 (75%) | 16/32 (50%) | 2/10 (20%) |
| No interstitial affection | 4/16 (25%) | 16/32 (50%) | 8/10 (80%) |
| echo-Doppler | |||
| Mean sPAP (mmHg) | 62 (n=16) | 58 (n=41) | 64 (n=11) |
| Mean VRT (m/s) | 2.38 (n=12) | 2.33 (n=27) | 2.24 (n=9) |
RFT: respiratory function tests; DLCO: carbon monoxide diffusion; CVF: forced vital capacity; PAP: pulmonary arterial pressure; ACA: anticentromere antibodies; Scl70: anti sclera 70 antibodies; echo-Doppler: Doppler echocardiography; sPAP: systolic pulmonary arterial pressure; VRT: tricuspid valve reflux speed; n: number of patients with available test results.
The average sPAP by ECHO at rest was 54.9±18mmHg, while the mean VRT was 3.3m/s. With respect to respiratory function tests, we found a mean DLCO of 45.7%±34% predicted, a FVC of 59.5%+27.8% predicted, and ratio of the FVC/DLCO of 1.3±0.6.
Using chest CT we found interstitial disease in 52% (30/58) of patients, and 28% (16/58) of patients had a limited form.
We calculated the Pearson correlation coefficient with the following variables FVC-sPAP (r=−0.040, P=.373), FVC/DLCO-sPAP (r=0.150, P=.149), DLCO-sPAP (r=−0.282, P=.01) and VRT-sPAP (r=0.658, P=.0001), demonstrating a linear association of moderate significance in relation to these 2 last variables (Figs. 1 and 2).
We also analyzed other variables such as the presence of distal ulcers (P=.184), the active capillaroscopy pattern (P=.241), the presence of antibodies to ACA (0.351) or Scl70 (P=.330), interstitial pulmonary involvement diagnosed by CT (P=.073) and its relationship to the severity of the PHT, although no statistically significant association with any of these variables was found.
There were 29 deaths (42.6%), of which PHT was the fundamental cause in 12 patients (Table 2). The years from diagnosis to death due to PHT were 1.8 years.
Causes of Death in Patients With SS and PHT.
| Cause of Death | No. of Patients, % |
| PHT | 12/29 (41%) |
| PHT+pulmonary fibrosis | 5/29 (17%) |
| Pulmonary fibrosis | 2/29 (7%) |
| PHT+renal crisis | 1/29 (3%) |
| Heart failure | 3/29 (10%) |
| Disseminated neoplasia | 2/29 (7%) |
| Liver cirrhosis | 3/29 (10%) |
| Renal crisis | 1/29 (3%) |
PHT, pulmonary arterial hypertension; SS, scleroderma.
In this study we observed a prevalence of PHT assessed by Doppler ultrasound of 20.5% and a correlation between increased values of pulmonary artery systolic pressure and decreased DLCO values with increasing VRT values statistically significant.
The prevalence found is similar to that described in other series.3–5 Thus, the study of Vonk et al.10 of the POEMS database found a prevalence of 9.9%; in the Launay et al.11 study it was 18.3% and in the UNCOVER study the prevalence was 26.7%. Probably, this variation is due to different diagnostic methods (Doppler and right heart catheterization), the different characteristics of the populations studied and the criteria used to define PHT.
By analyzing the values of PHT and DLCO levels, we observed that a decrease of 50% predicted higher numbers of pulmonary artery pressure. This is similar to that found by other authors.11,12
Mathai et al.13 found that the decrease in DLCO correlated PHBP in patients with interstitial lung disease, with the decrease in DLCO a marker of increased pulmonary pressure numbers. In the ItinérAIR-Sclérodermie14 study, it has been observed that a DLCO less than 60% predicted normal lung volumes associated with the echocardiographic diagnosis of PHT and that this decline may precede by several years the appearance of PHT. These data suggest that the decline in the numbers of DLCO is a predictor of the development of PHT, even in asymptomatic patients.
Other parameters to consider are VRT and sPAP. European guidelines for the diagnosis of PHT7 have been established arbitrarily to estimate the presence of VRT due to PHT and sPAP estimated by ECHO. Thus a VRT between 2.9 and 3.4m/s and sPAP between 37 and 50mmHg can be used as criteria to define a possible PHT.
Currently, PHT is the leading cause of mortality in these patients.11 Prior to the use of ACE inhibitors, renal crisis were the largest cause of mortality in patients with SS, but since the advent of these drugs, there has been a decrease in the number of newly affected renal patients.15
This study found a mortality of 42.6% among patients diagnosed with PHT and SS, with PHT itself the most common cause of death, followed by pulmonary fibrosis. These data are consistent with the ItenérAIR-Sclérodermie study, which showed a cumulative mortality of 3.04 per 100 patients/year with SS, with PHT the cause of death in 32.2%.14
The time elapsed from diagnosis of PHT and death found in this study was 1.8 years, similar to that found in other studies.1,2,4 MacGregor et al.16 reported that an sPAP measured by Doppler greater than or equal to 30mmHg was associated with increased mortality of 20%. Mukerjee et al.1 found that patients with sPAP>45mmHg had a mortality 30% higher at one year than patients with sPAP<32mmHg and that survival was 81, 63 and 56% at 1, 2, and 3 years, respectively, after the diagnosis of PHT.
The influence of PHT in the survival of these patients is especially important considering that, initially, PHT goes unnoticed and the first symptoms are often attributed generally to the underlying disease, delaying diagnosis and treatment. The detection of this complication in the early stages is therefore recommended, when treatment could prevent or halt its progression.
Thus sPAP and the VRT measured by Doppler echocardiograph, and DLCO values measured by spirometry, being non-lethal methods, are easily accessible, and may be useful as screening methods for PHT. However, it is important to note that the final confirmation of the diagnosis of this complication continues to be the performance of right cardiac catheterization.3,5,7,12
In this paper the detection of sPAP was made by echo-Doppler and not by right heart catheterization, which is a major limiting factor. However, major works, as exemplified by the EUSTAR6 group, on the causes of death and associated risk factors in patients with SS, used the Doppler method for the diagnosis of PHT. Another limiting factor that we must note is that this is a retrospective study and we did not study brain natriuretic peptide, which is currently used as a predictor of PHT.
In short, the PHT worsens the prognosis of patients with SS in the very short term. Therefore it is important to find predictors of onset, as early detection and treatment would help avoid much of both progression and the complications that this entails.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of Data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent. The authors have obtained the informed consent of the patients and /or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of InterestThe authors declare no conflict of interest.
Please cite this article as: Acosta Colmán MI, et al. ¿Podemos predecir la gravedad de la hipertensión arterial pulmonar en pacientes con esclerodermia? Reumatol Clin. 2012. http://dx.doi.org/10.1016/j.reuma.2012.03.007.