Infections in patients with systemic lupus erythematosus cause significant morbidity. Infection due to Listeria monocytogenes is considered an opportunistic disease, and has been published on rare occasions in patients with SLE.
ObjectiveTo review the presentation of listeria infections in the central nervous system (CNS) in SLE patients.
MethodologyWe conducted a literature review, selecting cases with central nervous system infection and confirmation of LM infection through culture.
ResultsTwenty six cases are described. The most common presentation was meningitis, with meningoencephalitis and brain abscesses being less frequent. The predisposing factors are: use of glucocorticoids, immunosuppressants, renal replacement therapy and the activity flares.
ConclusionCNS infection by listeria is rare and sometimes fatal. The atypical presentation may lead to a delay in diagnosis and appropriate treatment. L. monocytogenes should be included in the differential diagnosis of patients with SLE with neurological manifestations.
Las infecciones en pacientes con lupus eritematoso sistémico (LES) causan morbilidad significativa. La infección por Listeria monocytogenes (L. monocytogenes) se considera una enfermedad oportunista y ha sido publicada en raras ocasiones en pacientes con LES.
ObjetivoRevisar la forma de presentación de las infecciones por Listeria a nivel del sistema nervioso central (SNC) en pacientes con LES.
MetodologíaSe realizó una revisión bibliográfica, seleccionando los casos con cuadro de afección al sistema nervioso y confirmación de infección por L. monocytogenes en el cultivo.
ResultadosVeintiséis casos son descritos. La forma de presentación más común fue la meningitis, siendo la meningoencefalitis y los abscesos cerebrales menos frecuentes. Los factores predisponentes son: empleo glucocorticoides, inmunosupresores, terapia de sustitución renal y el brote de actividad.
ConclusiónLa infección del SNC por Listeria es poco común y, en ocasiones, fatal. La presentación atípica puede conducir a un retraso en el diagnóstico y el tratamiento adecuado. L. monocytogenes debe incluirse en el diagnóstico diferencial del paciente con LES y manifestaciones neurológicas.
Listeria monocytogenes (LM) is a Gram-positive rod widely found in nature. Human listeriosis is a rare disease with a global annual incidence of 0.2 to 7.4 million.1 In healthy populations, the highest rates of infection are seen in children aged one month and in adults over 60 years. Infections occurring outside the perinatal period happen in patients with hematologic malignancies, HIV infection or transplant patients or those treated with glucocorticoids (GC).2 The ingestion of contaminated food is the most common cause.3–5 In immunocompetent persons, infection produces self-limited diarrhea or flu, while in immunocompromised patients it may start as febrile gastroenteritis with bacteremia and subsequent seeding of bacilli to other organs. It shows tropism for the central nervous system (CNS), meningitis being the most common form of expression, although thromboencephalitis may be characteristic.4 CNS infection by LM has been published on rare occasions in patients with systemic lupus erythematosus (SLE)6–14 considered as of interest to the reader because it is included among the variety of differential diagnoses that should be considered in patients with SLE and neurological manifestations, as well as because its significant mortality. Given the rarity of this condition, we present a case diagnosed in our hospital as well as a review of the pathogenesis, clinical spectrum, diagnostic aids and treatment available, related to the topic.
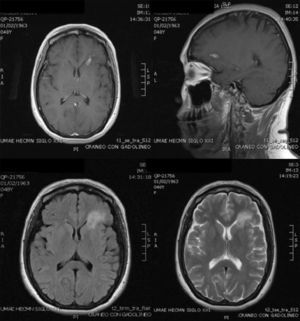
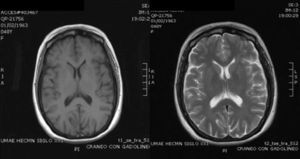
Case ReportThe patient was a 47-year-old woman diagnosed with SLE 10 years ago, with an outbreak of renal activity one year prior to admission that required treatment with mycophenolate mofetil (MMF) 3g daily, prednisone 1mg/kg with reduction to 0.5mg/kg/day, achieving disease activity remission. She was admitted with sudden onset headache, with an intensity of 9/10 on the visual analog scale, exacerbated with movement and no improvement with analgesics, accompanied by fever and nausea. Upon physical examination, she had no alterations in consciousness, impairment of higher mental functions or focal neurological data and presented negative meningeal signs. Study of the cerebrospinal fluid (CSF) showed leukocytes 27mm−3 (80% mononuclear), RBC 45mm−3, protein levels, normal CSF glucose, other tests and Gram stain were negative, IgM and IgG antibodies to cytomegalovirus and herpes virus were negative. Laboratory studies showed leukocytosis 13000cel./Mm3 with neutrophilia and monocytosis, low C3 levels with normal C4, elevated titers of anti-DNA, urinalysis with proteinuria, microhematuria and negative cylinders. The initial diagnosis was neuroinfection vs neuropsychiatric SLE activity; we began empirical treatment with ceftriaxone 2g every 12 h, vancomycin 1g every 12 h, acyclovir 600mg every 8 h, dexamethasone 8mg every 8 h. Magnetic resonance imaging (MRI) of the brain showed, on the frontal lobe, an injury consistent with a brain abscess (Fig. 1). At 48h the CSF was reported colorless, no leukocytes, erythrocytes 15, protein 39mg/dl and normal CSF glucose, PCR for M. tuberculosis was negative. After 4 days, LM growth was reported in the CSF, negative blood cultures, leading to a change in treatment ampicillin 2g intravenously every 4h for 6 weeks, with complete resolution without sequelae. A cerebral MRI after one month showed a nodular impregnation area 3mm diameter situated frontally with minimal adjacent edema. At 4 months there was evidence of the infectious process (Fig. 2).
MRI on admission: CSF spaces, cerebellum, medulla, pons, midbrain, corpus callosum and right hemisphere are normal. In the left hemisphere, at the level of the deep white matter of the frontal lobe, there is an area of hypo-hyperintense signal in T1 and T2, respectively, with an “edematous” bilobed appearance measuring approximately 17×6 mm, surrounded by vasogenic edema and that intensely permeates annularly after application of intravenous contrast. Changes are compatible with neuroinfection. Bilobed left frontal abscess.
A literature review was performed in Medline via Pubmed, with the following included as search terms: meningitis, brain abscess, lysteriosis, SLE, CNS infections. It was limited to articles published in English and Spanish. We selected all cases with CNS clinical symptoms and microbiological confirmation of infection with LM in the culture, and which possessed a case description.
ResultsWe found 26 described cases, of which 22 resulted in meningitis (85%), 2 in meningoencephalitis (8%) and 5 in cerebral abscess (19%) without any rhomboencephalitis case (Table 1). The most common presentations in patients with meningitis/meningoencephalitis were fever (90%), headache (78%), alteration of consciousness (70%), nausea (60%), vomiting (64%), meningeal signs (53%), diarrhea (50%) and focal neurological signs (41.6%). Patients with brain abscess had fever (100%), headache (100%), meningeal signs (33%), altered mental status (33%), nausea (100%), focal neurological signs (50%) and vomiting (33%) and in no case a history of diarrhea was reported (Table 2).
Demographic Data of 26 Patients With SLE and CNS Infection by Listeria monocytogenes.
| Author and reference | Case number | Year of publication | Gender | Age | Treatment | Diagnostics | Infection ype |
| Schulze et al.20 | 1 | 1953 | F | 19 | No steroids or immunosuppressive drugs | SLE | MeningitisBacteremia |
| Rosengarten and Bourn32 | 2. | 1959 | F | 48 | Steroids | SLE | MeningitisBacteremia |
| Harisdangkul et al.6 | 3. | 1992 | M | 31 | PNitrogenMustard | Active SLE. CRF in PD | Meningitis |
| Kraus et al.7 | 4. | 1994 | F | 25 | P (50mg/day) | Active SLE | Meningitis |
| 5. | 1994 | F | 29 | P (100mg/d) | Active SLE.Fisher-Evans syndrome | MeningitisBacteremia | |
| 6. | 1994 | F | 20 | No steroids or immunosuppressive drugs | Inactive SLECRF in HD | Meningitis | |
| 7. | 1994 | F | 28 | No steroids or immunosuppressive drugs | Inactive SLECRF in HD | Meningitis | |
| 8. | 1994 | F | 60 | P (7.5mg/day) | Inactive SLE | MeningitisBacteremia | |
| 9 | 1994 | M | 27 | P (200mg/day) | Active SLE | Meningitis | |
| Soga et al.10 | 10 | 1994 | F | 29 | NA | LESSLE | Meningitis |
| Mylonakis et al.30 | 11 | 1998 | F | 16 | Steroids, azathioprine and methotrexate | SLE | Meningitis |
| Eckburg et al.12 | 12 | 2001 | F | 34 | “Standard Immunosuppression” | SLERenal Transplantation | Brain abscessBacteremia |
| Lopez-Montes et al.28 | 13 | 2005 | F | 54 | CYC (750mg 2 doses). P (60mg/day) | SLE | Meningo-encephalitisBacteremia |
| Hung et al.15 | 14 | 2005 | F | 29 | CYC+PL | Active SLE | Meningitis |
| 15 | 2005 | F | 24 | PL | Active SLE | Meningitis | |
| 16 | 2005 | F | 22 | PL+HCQ | Active SLE | MeningitisBacteremia | |
| 17 | 2005 | M | 22 | PL+HCQ | Active SLE | Meningitis | |
| Hernandez-Belmonte et al.13 | 18 | 2008 | F | 60 | P (30mg/day)MMF (750mg/day) | Active SLE (hematologic) | Meningitis, brain abscess |
| Cone et al.14 | 19 | 2008 | F | 56 | P | SLE | Brain abscessBacteremia |
| Baizabal et al.18 | 20 | 2009 | F | 38 | NA | SLE | Meningitis, multiple brain abscesses |
| Tobon et al.11 | 21 | 2010 | F | 18 | P+AZA | SLE | MeningitisBacteremia |
| 22 | 2010 | F | 27 | P+MMF | SLE. CRF in HD | MeningitisBacteremia | |
| 23 | 2010 | F | 18 | P+MMF | LES | MeningitisBacteremia | |
| Lee et al.8 | 24 | 2011 | F | 32 | HCQ+MP+MMF | Active SLE | MeningitisBacteremia |
| McCaffrey et al.9 | 25 | 2012 | M | 57 | P (20mg/d) | LESSLE | MeningoencephalitisBacteremia |
| Case report | 26 | 2012 | F | 47 | P+MMF | Active SLE | MeningitisFrontal brain abscess |
AZA, azathioprine; CRF, chronic renal failure; CYC, cyclophosphamide; HCQ, hydroxychloroquine; HD, hemodialysis; IPD, Intermittent peritoneal dialysis; MMF, mycophenolate mofetil; NA, not available; P, prednisone; PL, prednisolone; SLE, systemic lupus erythematosus.
Clinical Features in 26 Patients With CNS Infection by Listeria monocytogenes.
| Case Number | Fever | Headache | Meningeal signs | Altered mental status | Diarrhea | Sickness | Vomiting | Focal neurological signs | Other manifestations |
| 1 | + | − | + | − | + | − | + | + | |
| 2 | + | + | + | + | + | − | + | + | Weakness, facial paralysis |
| 3 | ND | ND | ND | ND | ND | ND | ND | ND | |
| 4 | + | − | − | + | ND | ND | ND | ND | Petechiae |
| 5 | + | + | − | + | ND | ND | ND | ND | |
| 6 | − | + | + | + | ND | ND | ND | ND | |
| 7 | − | + | + | + | ND | ND | ND | ND | |
| 8 | + | + | − | + | ND | ND | ND | ND | |
| 9 | + | − | + | + | ND | ND | ND | ND | |
| 10 | + | + | + | ND | ND | ND | + | ND | |
| 11 | + | + | − | ND | ND | ND | ND | + | General discomfort for 14 days, difficulty walking, diplopia. Bilateral nystagmus. VII palsy. Weakness of the right arm |
| 12 | + | ND | ND | ND | ND | ND | ND | + | Parietal lobe brain abscess |
| 13 | + | ND | + | + | ND | ND | ND | ND | Bradypsychia |
| 14 | ND | ND | ND | ND | ND | ND | ND | ND | |
| 15 | ND | ND | ND | ND | ND | ND | ND | ND | |
| 16 | ND | ND | ND | ND | ND | ND | ND | ND | |
| 17 | ND | ND | ND | ND | ND | ND | ND | ND | |
| 18 | + | + | − | + | − | + | + | − | Right temporal abscess |
| 19 | ND | ND | ND | ND | ND | ND | ND | ND | Frontal and occipital brain abscess |
| 20 | + | + | + | − | − | + | − | + | Dysarthria, facial palsy, hemiparesis and hyperalgesia (Millard-Gubler syndrome) |
| 21 | + | + | − | − | − | − | + | − | Malaise |
| 22 | + | + | − | − | + | + | + | − | Myalgia, seizures |
| 23 | + | + | + | + | − | + | − | − | General malaise, joint pain, abdominal pain, photophobia, phonophobia, hydrocephalus |
| 24 | + | − | − | + | + | − | − | − | General malaise, nonproductive cough Acute renal failure requiring dialysis |
| Hydrocephalus | |||||||||
| 25 | + | + | + | + | + | + | + | − | Malar erythema |
| Hydrocephalus | |||||||||
| 26 | + | + | − | − | − | + | − | − |
(+), present; (−), absent; ND, not determined.
Pleocytosis was found in the CSF in 100% of the 12 cases where this information was available, ranging from 27 to 3664cel./Mm3. In 5 of 8 cases we predominantly found polymorphonuclear cells and mononuclear cells showed predominance in 3. 4 cases reported erythrocyte counts and all reported increased thereof. Protein levels were available in 7 cases, with elevated protein in 100% 116–399mg/dl and in 4 of 6 low CSF glucose levels. Gram staining was available in 5 cases, all negative. In 21 of 25 patients (84%) Listeria growth was reported in CSF culture and in 14 of 23 (61%) it was found in blood samples (Table 3). Three patients had meningitis not associated to the use of GC or immunosuppressants; one case was a patient with newly diagnosed SLE and the other 2 were on hemodialysis, without evidence of SLE activity. Information on treatment for meningitis/meningoencephalitis was was available in 21 cases and in 3 of 5 cases of brain abscess. In patients with meningitis/meningoencephalitis, 18 were treated with GC and 8 with cytotoxic drugs; in patients with brain abscess, 100% were treated with immunosuppressive medication, with a single patient requiring the use of prednisone, and 2 patients combining prednisone with MMF (Table 1).
Studies of Diagnosis and Treatment in 26 Patients With CNS Infection by Listeria monocytogenes.
| Study of cerebrospinal fluid | Others | Treatment | Outcome | |||||||
| Cells | Dif | Gluc, mg/dl | Prot, mg/dl | Gram | Cul | Hem | ||||
| 1 | L: 1430 | 85% PMN | 13mg | ND | ND | + | + | Leukocyte (18000 cel./Mm3) with neutrophilia | Streptomycin 500mg IM every 4 h | Died |
| 2. | L: 548E: 29 | ND | ND | ND | – | + | + | 2,000,000U penicillin+streptomycin 2g/day.Prednisone 7.5mg/daySulfadiazineChloramphenicol | Died | |
| 3. | ND | ND | ND | ND | ND | ND | ND | Resistant to cephalosporinsAmpicillin | Healed | |
| 4. | ND | ND | ND | ND | ND | + | − | Ampicillin+Amikacin | Died | |
| 5. | ND | ND | ND | ND | ND | + | + | Ampicillin | Healed | |
| 6. | ND | ND | ND | ND | ND | + | − | Ampicillin+Amikacin | Died | |
| 7. | ND | ND | ND | ND | ND | + | − | Ampicillin ceftriaxone+amikacin | Healed | |
| 8. | L: 534 | 79% PMN | ND | ND | ND | + | + | Normal CT | Ceftriaxone | Healed |
| 9 | ND | ND | ND | ND | ND | + | − | Ampicillin+Amikacin | Healed | |
| 10 | L: 3664 | ND | ND | 123 | ND | + | − | ND | Healed | |
| 11 | L: 1000 | 90% PMN | <25 | 365 | − | + | ND | Penicillin for 14 days | Survived with sequelae: diplopia, facial paresis | |
| 12 | L: 30 | Predominance ofPMN | ND | ND | ND | − | + | Penicillin for 4 weeks | Healed. Died at 4 months of acute hepatitis | |
| 13 | L: 196 | 95% MN | 30 | 160 | ND | + | + | Right temporal lobe encephalitis | Ampicillin 2 g every 4h for 6 weeks+gentamicin 200mg/day for 2 weeks | Improved without sequelae |
| 14 | ND | ND | ND | ND | ND | + | − | ND | Healed | |
| 15 | ND | ND | ND | ND | ND | + | − | ND | Died | |
| 16 | ND | ND | ND | ND | + | + | ND | Died | ||
| 17 | ND | ND | ND | ND | ND | + | − | ND | Healed | |
| 18 | L: 196E: 125 | 95% MN | 30 | 116 | ND | + | + | CT hypodense lesion in the right temporal lobe | Ampicillin 2g every 4h for 6 weeks+gentamicin 200mg/day for 2 weeks | Improved without sequelae |
| 19 | ND | ND | ND | ND | ND | + | + | ND | Healed | |
| 20 | ND | ND | ND | ND | ND | + | ND | MR multiple abscesses | Gentamicin for 2 weeks.TMP-SMX for 5 weeksAmpicillin for 12 weeks | Survived with sequelae: right facial hemiparesis, hypoalgesia in right hemisphere |
| 21 | ND | ND | ND | ND | ND | − | + | Elevated acute phase reactants | Ampicillin | Healed |
| 22 | ND | ND | ND | ND | ND | − | + | CSF supporting meningitis | Ampicillin | Healed |
| 23 | L: 600 | ND | ND | ND | ND | − | + | Elevated ESR and CRPHypocomplementemia | Ampicillin | Healed |
| 24 | L: 391 | ND | 301 | 552 | − | + | + | Elevated CRP | Ceftriaxone initially.Cultivation (+) at day 7 change to: ampicillin 2g every 8h plus gentamicin 100mg/day | Died |
| 25 | L: 3230E: 780 | 93% PMN | 2 | 399 | − | + | + | Elevation ESRLactic acid 24.7mmol/mL | Ampicillin 2g IV every 4h+ceftriaxone 2g IV every 12h | Healed |
| 26 | L: 27E: 45 | 80% MN | 61 | 396 | − | + | − | Ceftriaxone with vancomycin initially.Ampicillin 2g IV every 4h for 6 weeks | Improved without sequelae | |
Ampicillin, alone or in combination with an aminoglycoside, was the most widely used treatment (Table 3). The most common complication was hydrocephalus (n=3). 5 cases reported the presence of focal neurological signs, including 2 patients who died and another 2 who persisted with sequelae. One patient reported the cure of the infectious process without apparent sequelae, but died after 4 months with symptoms of acute hepatitis. A patient with meningitis persisted with diplopia and facial weakness; another patient progressed to meningitis and multiple brain abscesses presented and, as a complication, presented right facial hemiparesis and hypoalgesia of the right side of the body. The mortality rate was 27%.
DiscussionInfections remain a major cause of morbidity and mortality in patients with lupus because of the susceptibility to opportunistic infections due to immune dysfunction related to the disease or its treatment. CNS infections are not common in these patients, with an estimated prevalence of 0.53%–2.25%,15–18 with M. tuberculosis, C. neoformans and LM constituting the most common pathogens. Presenting symptoms may resemble a neuropsychiatric lupus flare, which makes diagnosis difficult. Wong et al. reported that 50% of episodes of neuropsychiatric SLE symptoms were caused by a systemic infection of the CNS.19
The association of Listeria and SLE has rarely been published in20,21 patients without steroid treatment and/or immunosuppressive drugs.7,11,15 Mook et al. reported a relative risk of Listeria meningitis in patients with systemic connective tissue diseases of 18.3 (12.6 to 26.6).22 Harisdangkul et al. reported that lysteriosis is uncommon in SLE and the most common form is bacteremia, found mainly in patients with renal failure and pregnancy, the latter constituting predisposing factors for lysteriosis in June.
The intracellular life cycle may explain the predilection for immunocompromised hosts. LM virulence allows it not only to penetrate into various cell types, but to avoid bactericidal mechanisms and survive in the cytoplasm of cells.23 It also has the ability to spread from cell to cell through pseudopodia formation within the infected cell, thus avoiding binding by antibodies. Resistance to infection is predominantly mediated by cellular immunity; their elimination requires T lymphocytes mediated cytotoxicity.
Controversy exists over whether SLE outbreaks may increase the risk of infection or not.18 In this review we found that 11 of the 26 cases had active SLE; in 12 cases the activity was not reported and only 3 patients had inactive SLE, all were on renal replacement therapy, suggesting that the number and function of T cells are affected especially during acute exacerbations of the disease, and may predispose to this infection, which is consistent with previous reports that have indicated that increased activity is associated to lymphopenia and increase the risk of infection.7,17,24
The tendency to intensive immunosuppressive therapy may increase cellular immune dysfunction, with an increased risk of infection18 GC were common predisposing factors in all cases (20 of 23 patients), leading to an alteration of cellular and humoral immunity. In animal models it has been shown that glucocorticoids increase susceptibility to infection with Listeria and cases of CNS infection with concomitant GC therapy in humans25 carry a poor prognosis.10 of 23 cases (43%) used concomitant immunosuppressive medication. The role of cytotoxic drugs as a risk factor is less clear than the use of GC. In a cohort of 174 patients, the most important risk factors were treatment with steroids and immunosuppressants, cytopenias and renal affection.11 Furthermore, the use of cytotoxic therapy was not a risk factor in other studies.6,17
Among the clinical manifestations, fever is the most common sign (90%–100%), regardless of immune status; headache is a nonspecific symptom and the presence of meningeal signs is variable and appears to be related to the degree of immunosuppression.26 In the series by Skoberg et al., neck stiffness was present in 26% of patients on immunosuppressive therapy, 47% of people with underlying disease without immunosuppressive therapy and in 54% of previously healthy subjects,27 which shows that Listeria meningitis cannot be excluded on the basis of the absence of meningeal signs.26 The presence of fever and headache is the rule in patients with brain abscess. Most had bacteremia, with the most common form of infection beinghematogenous spread, which is rare in brain abscesses caused by other processes that usually occur by direct extension from infected adjacent tissues. 60% was associated with meningitis and positive CSF cultures were present in 80%.
Because the clinical picture is nonspecific in patients with lupus and neurological, the study protocol should include an imaging study of the brain parenchyma and CSF study. Computed tomography (CT) and MRI add little to the diagnosis of meningitis, but they help exclude other causes of CNS. The most common feature of Listeria meningitis is a normal CT or hydrocephalus without focal lesions. MRI is superior to CT to demonstrate the involvement of the cerebral parenchyma.5
CSF findings in cases of meningitis are variable with a preponderance of polymorphonuclear leukocytes in almost 3/4 of the cases; the protein level is generally moderately elevated and glucose can be normal or low.23,26,28–30 In this review pleocytosis was found in 12 cases where the information was available, 63% with polymorphonuclear predominance. In the presence of lymphocytosis, CSF can simulate a viral meningitis and their presence does not exclude bacterial meningitis; antibiotic therapy should be given until culture results are available. Listeria encephalitis can mimic the herpes simplex virus encephalitis, particularly because both are one of the few causes of encephalitis associated with erythrocytes in CSF without CNS hemorrhage or traumatic spinal tap.5 In this series, 4 cases reported that erythrocytes in CSF were elevated. Gram staining shows a low diagnostic yield; no case was positive, and this was lower than what is reported in other conditions that show a 24%–50% positivity.23,31 The diagnosis requires isolation of LM. Cultures have a good diagnostic yield, and in 21 of 25 patients (84%) growth in the CSF is reported and in 14 of 23 (61%) the blood culture shows development, which is similar to other conditions with a reported positivity in CSF of 80%–95% and 60%–80% in9,26 blood cultures. However, early diagnosis cannot be performed due to the relatively slow growth of the microorganism, which takes approximately 3–4 days.9,32 Recently, it has been reported that lactic acid levels in CSF provide a key diagnostic test for early differentiation of acute bacterial meningitis (MBA) of non-bacterial meningitis, with an inexpensive test available. MBA patients present lactic acid levels ≥6mmol/L, whereas those with a viral or aseptic meningitis lactic acid level are <3mmol/L.33–41 Another test for a rapid detection is the realization of real-time PCR-Hly, but this test is not available in clinical practice.42 The diagnostic difficulty resides in the distinction between a CNS infection and exacerbations of neuropsychiatric SLE disease activity, which is a diagnostic and therapeutic challenge, since both can coexist.43 McCaffrey et al. reported early diagnosis of Listeria meningoencephalitis in a patient with lupus by demonstrating a high level of lactic acid in CSF, days before the CSF culture and blood cultures were positive.9
Penicillin and ampicillin44 constitute the initial treatment. It is considered that the antibiotic of choice in bacteremia or meningitis is ampicillin at a meningeal dose (2 g every 4h), since it crosses the brain barrier better than penicillin. Since its bactericidal activity is slow, we recommend the coadministration with an aminoglycoside. Penicillins with gentamicin act synergistically against Listeria both in vitro and in vivo, and therefore it has become the standard of care. However, the advantages of combination therapy have never been shown in prospective clinical trials. In a retrospective study, combination therapy was not superior to monotherapy with penicillin.23 Combination therapy with gentamicin is recommended in the treatment of CNS Listeria infections, endocarditis, and infections in immunocompromised patients, such as patients with SLE.28 In patients allergic to penicillin or in whom a second line treatment is recommended cotrimoxazole is an option, being bactericidal against this organism, with its 2 components having excellent CNS penetration and allowing to be administered orally in doses of 320/1600mg every 8–12h.4,26 The isolates are generally susceptible to vancomycin in vitro and clinical success with vancomycin have been reported, including our case. However, a case of Listeria meningitis has been described fdeveloped d uring45 vancomycin therapy. In general, there is insufficient information on the clinical use of vancomycin for listeriosis. In a series of patients with severe Listeria meningoencephalitis, the combination of ampicillin and cotrimoxazole gave better results in terms of relapses and neurological sequelae than ampicillin with gentamicin and found no difference in mortality between patients treated with ampicillin or penicillin monotherapy and those receiving combined treatment with beta-lactam plus aminoglycoside (73% vs 70%, P>0.05).23 In cases of brain abscess, surgical intervention may not be necessary. Numerous case reports describe successful treatment with antimicrobial therapy alone.5 There are no randomized trials to determine whether there is a superior treatment regimen, but clinical experience dictates that ampicillin should remain the drug of choice.46 The optimal duration of treatment is unknown; with relapses reported in patients with meningitis treated for less than 2 weeks. We suggest extending treatment for meningitis in immunocompromised patients for at least 4–8 weeks, with no less than 5–6 weeks of ampicillin and gentamicin for 2–4 weeks.5,8,26 Patients with brain abscess, encephalitis or rhomboencephalitis should be treated for at least 6 weeks, followed by brain imaging studies. Mylonakis et al. recommend that the total duration of treatment should be individualized according to clinical response and clinical and radiological monitoring is essential before deciding on the end of treatment.4,30
Mortality remains high despite antibiotics and studies consistently report rates of 20%–50% among those who received therapy, especially those with underlying diseases, old age, immunosuppression associated to severe sepsis and 1.15 CNS infection.1,15,23,47,48 It is considered that the incidence will increase due to the increased life expectancy of immunosuppressed patients. The prognosis apparently depends not only on listeriosis itself, but also on the type and stage of the underlying disease; there is a variation in mortality rates with different diseases, 48% in patients with cancer, 29% in patients with iatrogenic diseases and less than 20% in previously healthy patients.26,29,49
Clinical suspicion, along with early diagnosis and correct treatment in the early hours of symptoms would be measures that may prevent high mortality.15 Aronin et al. reported better results in patients with early treatment.26 In this series there were no relapses in patients who survived the infection and the onset of focal neurologic signs worsened the prognosis.
ConclusionsCNS infection by Listeria in patients with SLE is rare and sometimes fatal; an atypical presentation may lead to a delay in diagnosis and initiation of appropriate treatment. Therefore, it is suggested that an imaging study, a blood culture and CSF examination, including lactic acid levels and cultures of bacteria, mycobacteria and fungi be undertaken. The use of GC, immunosuppressants, renal replacement therapy and the outbreak of activity are factors that predispose to the development of CNS listeriosis in patients with lupus. LM should be included in the differential diagnosis of these patients, having to consider initiating empiric treatment with ampicillin due to the high resistance to cephalosporins, considered as the treatment of choice in patients with CNS infection.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that experiments have not been performed on humans or animals.
Data confidentialityThe authors state that no patient data appear in this article.
Right to privacy and informed consentThe authors have obtained the informed consent of patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Conflict of InterestThe authors declare no conflict of interest.
Please cite this article as: Horta-Baas G, Guerrero-Soto O, Barile-Fabris L. Infección del sistema nervioso central por Listeria monocytogenes en pacientes con lupus eritematoso sistémico: análisis de 26 casos, incluyendo el reporte de un caso nuevo. Reumatol Clin. 2013;9:340–347.