The biological drugs that target tumour necrosis factor α (TNFα) are now widely used to treat rheumatoid arthritis (RA) and other immunomediated inflammatory diseases. Although they have been shown to be highly effective and to have a good safety profile,1 they are also associated with adverse cutaneous events, including psoriasis and psoariasiform lesions.2 We present a patient diagnosed RA who developed palmoplantar pustulosis (PPP) during treatment with certolizumab pegol (CZP), and we also review the published cases of psoriasis induced by this drug.
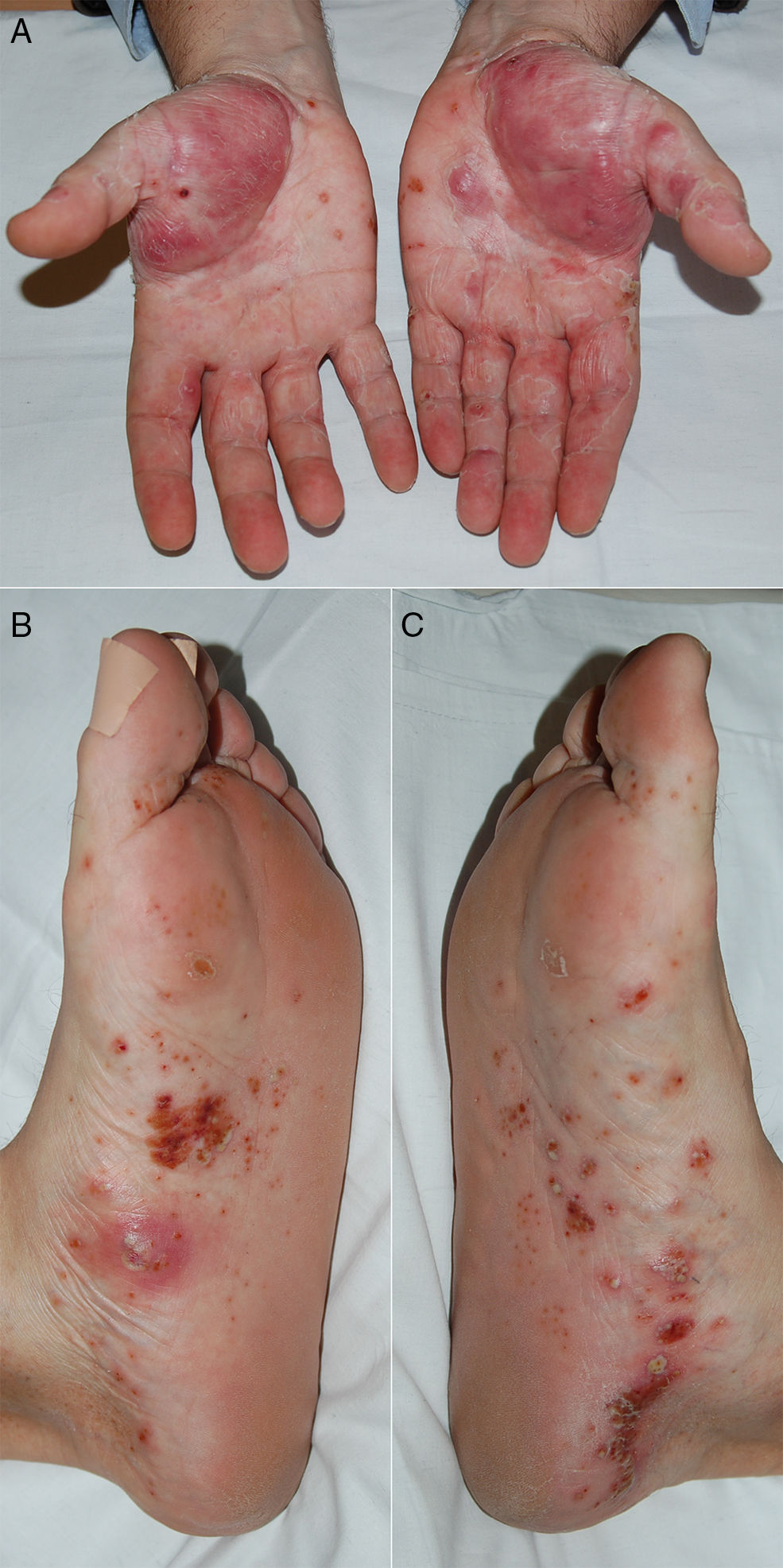
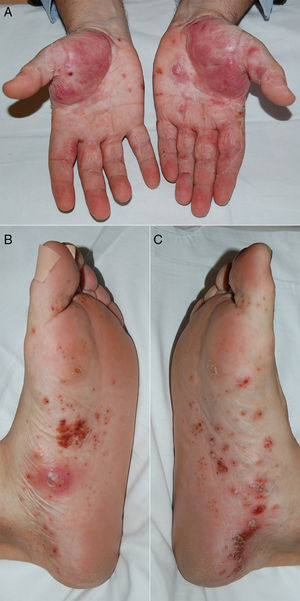
A 57 year-old male with a history of diabetes mellitus and arterial hypertension was diagnosed RA at the age of 50 years-old due to his polyarthritis in the small joints of the hands, raised acute phase reagents and positivity for rheumatoid factor and anti-cyclic citrullinated peptide antibodies. He was treated at first with methotrexate (maximum dose 20mg/week, subcutaneous), with clinical improvement and good tolerance. However, after 5 years he underwent gradual worsening of the inflammatory symptoms in the hands and persistently high levels acute phase reagents (disease activity measured by DAS28: 5). Due to this it was decided to add CZP (subcutaneous 200mg every 2 weeks), achieving a good response in the joints and analytical results within the first 2 months. After 3 months of anti-TNFα treatment he visited the emergency department due to a sudden outbreak of converging and painless millimetrical pustular lesions on the palms and soles of the feet (Fig. 1), with no signs of infection or lesions in other locations. Biopsy of a palm lesion was histopathologically compatible with PPP. CZP was withdrawn, but methotrexate was maintained, starting topical corticoid therapy with betamethasone cream. The latter was subsequently changed to clobetasol cream with occlusive bandages and laser sessions, and the lesions disappeared completely 4 months after suspending the anti-TNFα. The patient remained in remission after a 6-month observation period, continuing treatment only with subcutaneous methotrexate 25mg/week, without the need to recommence the biological treatment.
The appearance of psoriasis de novo or worsening of pre-existing psoriasis is an AE associated with all anti-TNFα drugs, and it may occur at any time (from days until years after the start of treatment), without any variations according to sex or age.2,3 Although it has been described in almost all of the diseases treated with anti-TNFα, up to 75% of cases correspond to inflammatory rheumatological disease.2 A recent meta-analysis that included 216 cases of de novo psoriasis induced by anti-TNFα found the following frequency for each of the following drugs: infliximab 62%, adalimumab 21.8%, etanercept 14.4%, CZP 1% and golimumab 0.5%.3 Rather than reflecting any difference between these drugs, this may be due to the higher number of patient-years of exposure to the first anti-TNFα approved, in comparison with CZP and golimumab, and it is now considered to be a class effect of these drugs.2,3
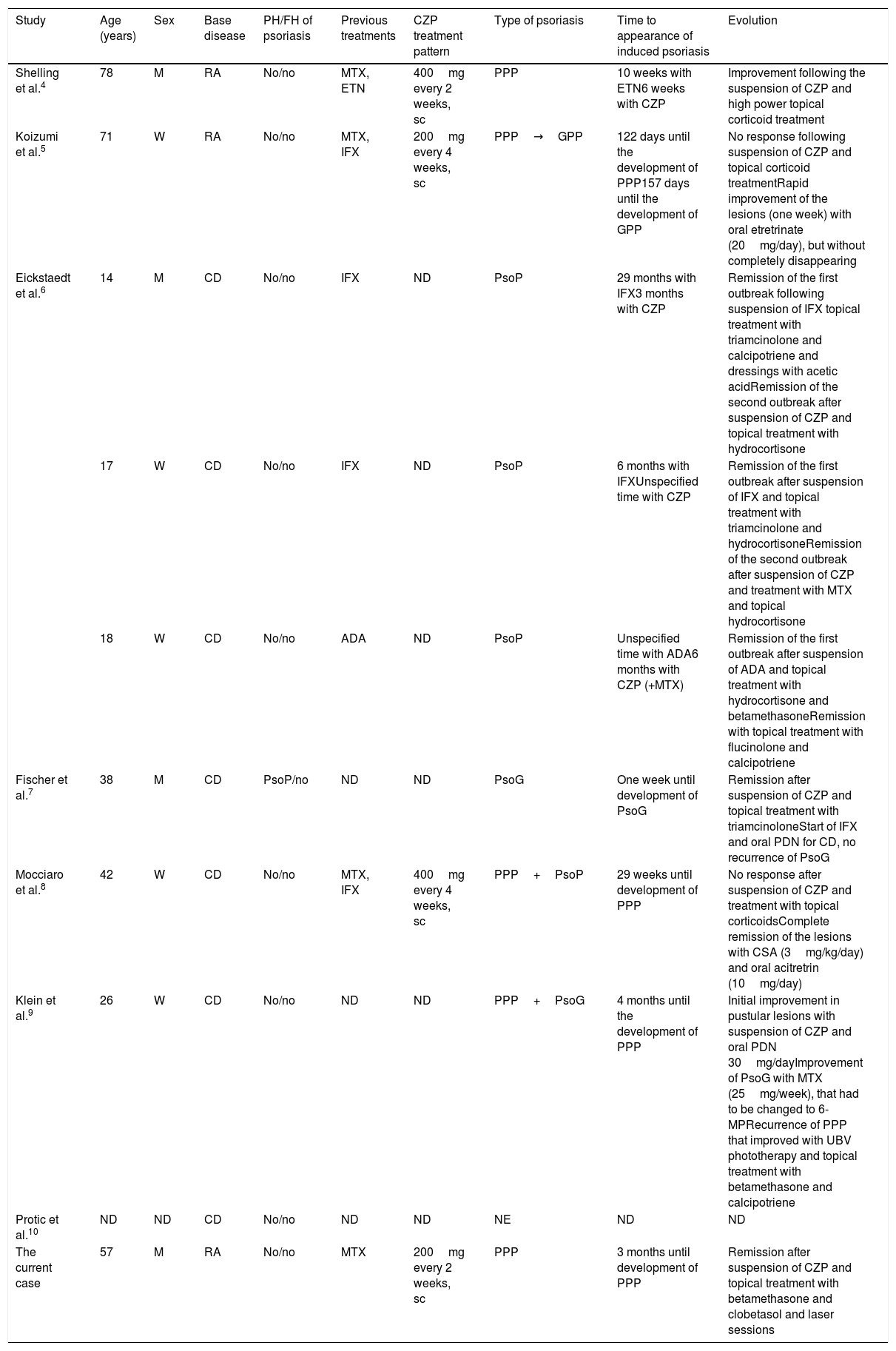
In our review of the literature (PubMed) we found 9 cases of CZP induced psoriasis, 2 in RA and 7 in Crohn's disease (Table 1). The cases which occurred in RA corresponded to PPP; in the first of these it was a recurrent AE (the first event after etanercept),4 while in the second it appeared de novo and progressed to generalised pustular psoriasis (GPP).5 CZP was withdrawn in both cases and after dermatological treatment the patients showed good clinical evolution of the lesions. All of the cases which arose in Crohn's disease were de novo (3 with CZP as the first anti-TNFα and 3 in which it was the second) and the types of psoriasis they had were: plaque psoriasis (3),6guttata psoriasis (1),7 PPP+plaque psoriasis (1),8 PPP+guttata psoriasis (1)9 and one unspecified type (1).10 In 5 cases CZP was suspended, 4 cases had a good response to topical corticoid therapy and 2 cases also required photochemotherapy and acitretrin.
Characteristics of the Patients With Certolizumab-induced Psoriasis.
| Study | Age (years) | Sex | Base disease | PH/FH of psoriasis | Previous treatments | CZP treatment pattern | Type of psoriasis | Time to appearance of induced psoriasis | Evolution |
|---|---|---|---|---|---|---|---|---|---|
| Shelling et al.4 | 78 | M | RA | No/no | MTX, ETN | 400mg every 2 weeks, sc | PPP | 10 weeks with ETN6 weeks with CZP | Improvement following the suspension of CZP and high power topical corticoid treatment |
| Koizumi et al.5 | 71 | W | RA | No/no | MTX, IFX | 200mg every 4 weeks, sc | PPP→GPP | 122 days until the development of PPP157 days until the development of GPP | No response following suspension of CZP and topical corticoid treatmentRapid improvement of the lesions (one week) with oral etretrinate (20mg/day), but without completely disappearing |
| Eickstaedt et al.6 | 14 | M | CD | No/no | IFX | ND | PsoP | 29 months with IFX3 months with CZP | Remission of the first outbreak following suspension of IFX topical treatment with triamcinolone and calcipotriene and dressings with acetic acidRemission of the second outbreak after suspension of CZP and topical treatment with hydrocortisone |
| 17 | W | CD | No/no | IFX | ND | PsoP | 6 months with IFXUnspecified time with CZP | Remission of the first outbreak after suspension of IFX and topical treatment with triamcinolone and hydrocortisoneRemission of the second outbreak after suspension of CZP and treatment with MTX and topical hydrocortisone | |
| 18 | W | CD | No/no | ADA | ND | PsoP | Unspecified time with ADA6 months with CZP (+MTX) | Remission of the first outbreak after suspension of ADA and topical treatment with hydrocortisone and betamethasoneRemission with topical treatment with flucinolone and calcipotriene | |
| Fischer et al.7 | 38 | M | CD | PsoP/no | ND | ND | PsoG | One week until development of PsoG | Remission after suspension of CZP and topical treatment with triamcinoloneStart of IFX and oral PDN for CD, no recurrence of PsoG |
| Mocciaro et al.8 | 42 | W | CD | No/no | MTX, IFX | 400mg every 4 weeks, sc | PPP+PsoP | 29 weeks until development of PPP | No response after suspension of CZP and treatment with topical corticoidsComplete remission of the lesions with CSA (3mg/kg/day) and oral acitretrin (10mg/day) |
| Klein et al.9 | 26 | W | CD | No/no | ND | ND | PPP+PsoG | 4 months until the development of PPP | Initial improvement in pustular lesions with suspension of CZP and oral PDN 30mg/dayImprovement of PsoG with MTX (25mg/week), that had to be changed to 6-MPRecurrence of PPP that improved with UBV phototherapy and topical treatment with betamethasone and calcipotriene |
| Protic et al.10 | ND | ND | CD | No/no | ND | ND | NE | ND | ND |
| The current case | 57 | M | RA | No/no | MTX | 200mg every 2 weeks, sc | PPP | 3 months until development of PPP | Remission after suspension of CZP and topical treatment with betamethasone and clobetasol and laser sessions |
ADA: adalimumab; FH: family history; PH: personal history; RA: rheumatoid arthritis; CSA: cyclosporine A; CZP: certolizumab pegol; CD: Crohn's disease; ETN: etanercept; M: man; IFX: infliximab; W: woman; MTX: methotrexate; ND: no available data; NE: not specified; GPP: generalised pustular psoriasis; PPP: pustulosis palmoplantar; PsoG: psoriasis guttata; PsoP: plaque psoriasis; sc: subcutaneous; UBV: ultraviolet B light.
The types of anti-TNFα-induced psoriasis described the most often in the literature are: plaques 44.8%, PPP 36.3%, GPP 10.9% and guttata 8%.3 The high frequency with which PPP occurs in patients treated with anti-TNFα in comparison with the general population (an incidence of 0.12%) suggests that this is a specific AE of these drugs. Although suspension of the treatment is not always indispensable, severe forms such as PPP and GPP may respond better if the anti-TNFα is withdrawn.6
To conclude, CZP may be associated with the development of induced psoriasis, as is the case with other anti-TNFα drugs, regardless of their indication, and PPP is one of the most frequent forms of presentation.
Please cite this article as: Villalobos-Sánchez L, Larena-Grijalba C, Alía-Jiménez A, Sifuentes-Giraldo WA. Pustulosis palmoplantar inducida por certolizumab pegol: presentación de un caso y revisión de la literatura. Reumatol Clin. 2019;15:e163–e165.