The prevalence of rheumatoid arthritis (RA) has been reported to be 1.8% in patients with primary biliary cholangitis (PBC), and mitochondrial antibodies (AMA) have been found in 10% of RA patients.1,2 It was reported that T and B cells play an important role in the inflammatory process of both RA and PBC.3,4 The potential therapeutic target is determined by the pathogenetic mechanism. We report a case series of patients with RA and PBC with good clinical response to rituximab.
The first case was a 61-year-old female with the diagnosis of RA and PBC. The serological tests were negative, except for rheumatoid factor (RF), anti-cyclic citrullinated peptide antibodies (anti-CCP) and AMA-M2. Serum lipids, glucose, renal function tests, parathyroid-thyroid hormones, viral markers and protein electrophoresis pattern were normal. Abdominal ultrasonography showed no pathology. She had a medical history of smoking and receiving ursodeoxycholic acid (15mg/kg/day), methotrexate (MTX, 15mg/week), hydroxychloroquine (200mg/day) and methylprednisolone (4–8mg/day). Due to high disease activity (DAS-28: 6.24) rituximab (1000mg IV every 2 weeks) was administered.
The second case was a 68-year-old female with the diagnosis of RA and PBC. She had medical history of using ursodeoxycholic acid (10mg/kg/day), leflunomide (20mg/day), hydroxychloroquine (200mg/day) and prednisone (10mg/day). She had a RF of 34.2U/mL (3–18IU/mL) and anti-CCP of 8.7U/mL (0–4.99U/mL). AMA-M2 was positive and viral serology was negative. Abdominal ultrasonography showed no pathology. Due to exacerbation of arthritis and high disease activity, rituximab (1000mg IV every 2 weeks) was administered.
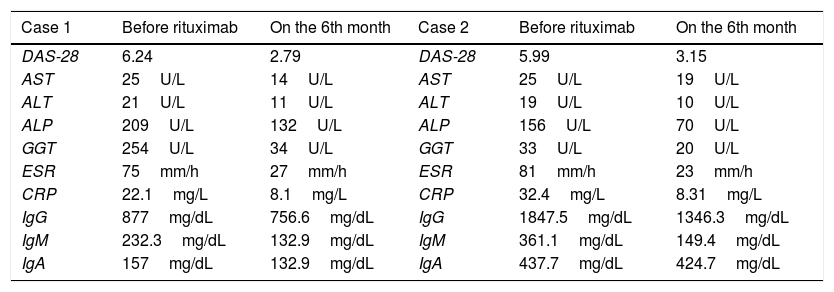
Changes in laboratory findings of 2 cases are shown in Table 1.
Laboratory features of patients with rheumatoid artritis and primary biliary cholangitis.
| Case 1 | Before rituximab | On the 6th month | Case 2 | Before rituximab | On the 6th month |
|---|---|---|---|---|---|
| DAS-28 | 6.24 | 2.79 | DAS-28 | 5.99 | 3.15 |
| AST | 25U/L | 14U/L | AST | 25U/L | 19U/L |
| ALT | 21U/L | 11U/L | ALT | 19U/L | 10U/L |
| ALP | 209U/L | 132U/L | ALP | 156U/L | 70U/L |
| GGT | 254U/L | 34U/L | GGT | 33U/L | 20U/L |
| ESR | 75mm/h | 27mm/h | ESR | 81mm/h | 23mm/h |
| CRP | 22.1mg/L | 8.1mg/L | CRP | 32.4mg/L | 8.31mg/L |
| IgG | 877mg/dL | 756.6mg/dL | IgG | 1847.5mg/dL | 1346.3mg/dL |
| IgM | 232.3mg/dL | 132.9mg/dL | IgM | 361.1mg/dL | 149.4mg/dL |
| IgA | 157mg/dL | 132.9mg/dL | IgA | 437.7mg/dL | 424.7mg/dL |
DAS-28, disease activity score-28; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gammaglutamyltransferase.
Hepatic involvement is rare, and not common in RA. Hepatic abnormalities are generally associated with hepatotoxic drugs, viral hepatitis and alcoholic cirrhosis.5,6 AMA, which have an important role in PBC pathogenesis, are useful indicators for liver diseases (PBC) in RA. B-cells, T-cells, and TNF-α have been implicated in the pathogenesis of PBC.3,4 B-cells may contribute to pathogenesis with immunoglobulin production, bile ducts destruction and regulatory function. It suggests that B-cells and immunoglobulins are important and B-cell depletion may be a promising therapy for PBC.3,4 Lazrak et al. reported combination therapy with MTX and rituximab in a 60-year-old RA and PBC patient.7 There was a good clinical response for RA, but abnormal liver function tests were found on the 5th month of therapy. Exacerbation and development of PBC with B cell depletion in mice was reported.8,9 Tsuda et al. reported 6 PBC patients treated with rituximab due to the suboptimal response to UDCA.3 The study showed that rituximab reduced B cells, AMA, alkaline phosphatase, IgG, IgM, and IgA. It has been reported the improvement in liver laboratory parameters with rituximab, but not the improvement in symptoms such as fatigue. According to Myers et al., the levels of ALP, IgM, and AMA were significantly decreased at 6 months of rituximab in patients with PBC and incomplete response to ursodeoxycholic acid.10 At 12 months of treatment, the fatigue was the same, and 60% of patients had a reduction in itching. We described 2 cases with RA and PBC who had a good clinical response to rituximab. The activity of both diseases and abnormal hepatic tests returned to normal levels after therapy. However, reduction in the progression of disease and cirrhosis is unclear.
Clinicians should be aware of hepatic diseases in patients with RA. It should be kept in mind that AMA is useful for the diagnosis of PBC in RA patients with abnormal hepatic tests. Rituximab was tolerable in RA patients with PBC. However, further studies in larger groups are needed to clarify its role and long-term efficacy in PBC.