Pulmonary hemorrhage (PH) occurs in 2%–5% of SLE patients, and is associated with a high mortality rate (79%–90%). Diagnostic criteria for this complication include: (1) pulmonary infiltrates, with at least ¾ of lung tissue involved in a chest X-ray, (2) acute respiratory failure, (3) a decrease of 3g/dl or more in hemoglobin levels. PH might lead to organized pneumonia, collagen deposition, and pulmonary fibrosis which in time might cause changes in pulmonary function tests with either restrictive or obstructive patterns.
AimTo evaluate the existence of abnormalities in pulmonary function tests after a PH episode.
MethodsWe included patients with SLE and primary vasculitis that developed PH. During the acute episode, we measured SLEDAI in SLE patients, five factor score in microscopic polyangiitis (MPA) and Birmingham Vasculitis Activity Store (BVAS) in granulomatosis with polyangiitis (GPA) (Wegener). We determined the number of PH events, treatment, and ventilator assistance requirements and correlated its association with abnormal pulmonary function tests.
ResultsWe included 10 patients, 7 with SLE, 2 with MPA and 1 with GPA (Wegener). The mean activity measures were: SLEDAI 20.4±7.5, FFS 2, and BVAS 36. Treatment consisted in methylprednisolone (MPD) in 3 patients, MPD plus cyclophosphamide (CY) in 6 patients, and MPD, CY, IV immunoglobulin, and plasmapheresis in one patient. Five patients required ventilatory support. We found abnormalities in pulmonary function tests in 8 patients, three had an obstructive pattern and five a restrictive pattern; 2 patients did not show any change. We did not find a significant association with any of the studied variables.
ConclusionPH might cause abnormalities in pulmonary function tests and prolonged immunosuppressive treatment could be required.
La hemorragia pulmonar (HP) se presenta en el 2 al 5% de los pacientes con lupus eritematoso sistémico (LES) y puede alcanzar una mortalidad del 70 al 90%. Los criterios para el diagnóstico de HP son: a) infiltrados alveolares en 3 cuartas partes de los campos pulmonares en la radiografía de tórax; b) insuficiencia respiratoria de inicio agudo, y c) descenso de la hemoglobina >3g/dl. La HP puede conducir a neumonía organizada, depósito de colágeno en vías aéreas pequeñas y, consecuentemente, fibrosis pulmonar, lo cual puede alterar la función pulmonar con cambios obstructivos o restrictivos.
ObjetivoEstablecer, mediante pruebas de funcionamiento respiratorio (PFR), si existen alteraciones en la función respiratoria después de haber presentado una HP.
MétodosSe incluyó a pacientes con LES o con vasculitis primaria que presentaron HP. En el momento de la HP, se determinó actividad con SLEDAI para los pacientes con LES, «five factor store» (FFS) para poliangitis microscópica (PAM) y «Birmingham Vasculitis Activity Store» (BVAS) para granulomatosis con poliangitis (GP) (Wegener). Se determinaron el número de eventos de HP, el tratamiento utilizado y el requerimiento de asistencia mecánica ventilatoria (AMV) para determinar su probable asociación con las alteraciones de la función respiratoria medidas por pletismografía y/o espirometría.
ResultadosSe incluyó a 10 pacientes, 7 con LES y 3 con vasculitis primaria (2 con PAM y uno con GP (Wegener). La media±desviación estándar de SLEDAI fue de 20,4±7,5, la de FFS 2 y la de BVAS 36. Un paciente presentó 2 episodios de HP y otro 5. El tratamiento fue metilprednisolona (MPD) en 3 pacientes, MPD más ciclofosfamida (CFM) en 6 pacientes y MPD, CFM, inmunoglobulina por vía intravenosa y plasmaféresis en un paciente. Cinco pacientes requirieron AMV. Se encontró disfunción pulmonar en 8 pacientes; 3 tuvieron patrón obstructivo y 5 patrón restrictivo; 2 tuvieron PFR normales. No se encontró asociación significativa entre las variables y las alteraciones de la función respiratoria.
ConclusiónLa HP causa alteraciones de la función respiratoria en un alto porcentaje de pacientes y es probable que se requiera tratamiento inmunosupresor a largo plazo una vez resuelto el episodio agudo.
Pulmonary hemorrhage (PH) or diffuse alveolar hemorrhage is a clinical and pathological syndrome that puts the patients life at risk; it may present as a single or recurrent episode and may lead to organized pneumonia and collagen deposition in the small airway with the development of lung fibrosis.1,2
There are several causes for PH and most of them share the same physiopathogenic mechanism, namely damage to the alveolar microcirculation with local hemorrhage.3 Histologically, it presents as lung capillaritis characterized by the infiltration of neutrophils in small caliber vessels, interstitial erythrocytes, hemosiderin deposits and septal alveolar capillary occlusion4; although this capillaritis is not pathognomonic, it usually points to the presence of underlying systemic vasculitis.5 In patients with rheumatic disease, HP may manifest as a primary manifestation of disease activity; the most commonly associated diseases are systemic lupus erythematosus (SLE) and primary systemic vasculitidies.6
PH occurs in SLE in 2%–5% of cases and represents 3.7% of hospital admissions due to SLE activity, with a mortality of 70%–90%.7–9 In 1985, Abud-Mendoza et al.10 published the criteria to diagnose PH: (a) dense alveolar infiltrates in ¾ or more of the lungs in the X-ray; (b) acute onset respiratory distress, and (c) a descent >3g/dl in hemoglobin; this was confirmed by Barile et al.,11 who included 34 patients with SLE associated PH, who had as the most common manifestations: hemoglobin descent (91.17%), respiratory distress (100%), hemoptysis (58.82%), X-ray infiltrates (100%) and hypoxemia (82.35%), with the important observation that 14 of the patients did not present hemoptysis (41%). In patients without the classic triad for PH, computed tomography (CT) images may confirm the diagnosis in a significant percentage of patients. In a study by Cortese et al., the X-rays of 20 patients were reviewed and compared to CT, with the latter being superior to conventional X-rays for the detection of ground glass opacities. The greater usefulness of this diagnostic method was emphasized in cases of suspected PH with a normal chest X-ray with or without the classic PH triad.12–14
Bronchoscopy with bronchoalveolar lavage is another valuable test for the diagnosis of PH, which allows the confirmation of PH, in addition to being useful in searching for the origin of the bleeding.15,16 This study can even be performed in patients with assisted mechanical ventilation (AMV), something that is commonly employed in patients with respiratory distress secondary to PH as a measure of vital support. AMV increases survival in these patients, although it may leave secondary lung dysfunction due to areas of healing, of variable severity, which may be completely resolved or not during its progression.
Regarding treatment of PH, there is no established consensus. Different treatment regimes have been proposed; the most common employs pulse methylprednisolone, 1g/day (up to 5 days in some cases) and cyclophosphamide, with variable results. Plasmapheresis is controversial; however it has proven useful in patients with vasculitis associated to anti-neutrophil cytoplasm antibodies and in patients with SLE who are not responding to conventional treatment with steroids.17,18
PH may cause interstitial fibrosis and this may impact pulmonary function, with restrictive or obstructive changes, leading to parenchymal and small airway damage, leading to bronchiolitis and enphysema.19,20 With this in mind we sought to establish the alterations in respiratory function after PH in patients with SLE and primary vasculitis through a ventilator-perfusion scan because this study allows us to evaluate the degree and type of dysfunction of the respiratory system through the measurement of forced vital capacity (FVC), forced expiratory volume in the first second (FEV1) and the FEV1/FVC ratio or Tiffeneau index.
The objectives of this study were to establish, through a ventilator-perfusion scan or spirometric test, the prevalence of respiratory function alterations and determine the factors associated with this dysfunction in patients with SLE and primary systemic vasculitis after a PH.
Patients and MethodsPatients who had PH demonstrated by a triad of acute respiratory distress, a descent of >3g/dl of hemoglobin and diffuse alveolar infiltrates in ¾ of the lung as seen in the X-ray from the Hospital de Especialidades del Centro Médico Nacional Siglo XXI with SLE or primary systemic vasculitis, or those in whom the diagnosis was confirmed by high resolution computed tomography or bronchoscopy were included.
We included patients over 18 years of age who complied with the classification criteria proposed by the American College of Rheumatology for SLE21 or the Chapel Hill criteria for primary systemic vasculitis,22,23 and in whom the measurement of lung function through ventilator-perfusion scans or spirometry was possible in order to determine the residual volume, the total lung capacity (TLC), the residual functional capacity, FVC, FEV1and the FEV1/FVC ratio or Tiffeneau index, in addition to the degree of obstruction and lung restriction.
The SLEDAI and SLICC indexes were measured in SLE and the five factor score (FFS) was used for microscopic polyangiitis (PAM) and BVAS in granulomatosis with polyangiitis (GP) (Wegener), in order to establish the degree of activity at the moment of the PH; the number of PH events were determined as was the type of treatment, the requirement for AMV and the factors associated to chronic respiratory dysfunction.
Study DesignRetrospective, transversal, analytic.
Statistical AnalysisDescriptive statistics and bivariate analysis with a chi squared test, Fisher's exact test and OR (odds ratio) with 95% confidence intervals (CI 95%) were performed; a P<.05 was considered as statistically significant. We used the SPSS 15.0 software package.
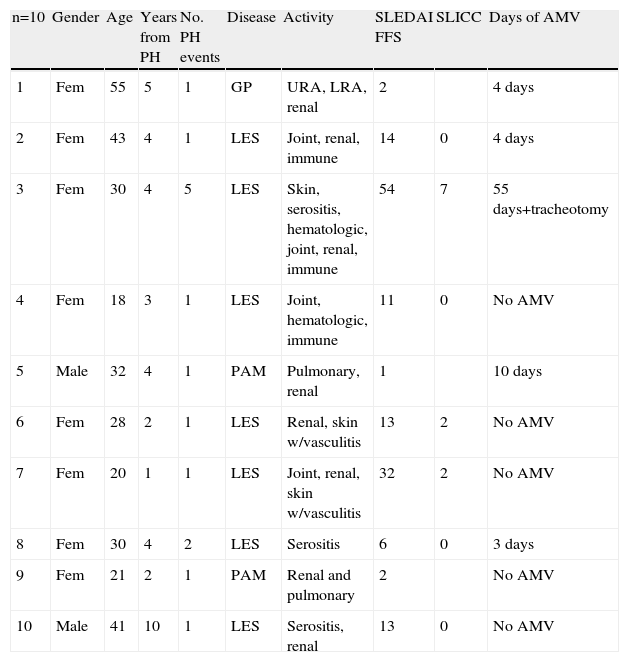
ResultsA retrospective analysis of patients with SLE and systemic vasculitis was performed from September 1998 to November 2007, identifying 11 patients with PH who had survived, of whom 10 were contacted and underwent lung function tests with their prior informed consent. One patient was not found. The rest were included for analysis. 3 had systemic vasculitis: one with GP (Wegener) and positive anti-neutrophil cytoplasm antibodies and 2 with PAM and positive anti-neutrophil perinuclear cytoplasm antibodies; the rest (7 patients) had a diagnosis of SLE. Mean age of patients at the moment of the study was 30 years (ranging from 18 to 43 years old) for SLE and 35 (range 21–55) for those with systemic vasculitis. Baseline characteristics are shown in Table 1.
Baseline Characteristics of the Patients.
| n=10 | Gender | Age | Years from PH | No. PH events | Disease | Activity | SLEDAI FFS | SLICC | Days of AMV |
| 1 | Fem | 55 | 5 | 1 | GP | URA, LRA, renal | 2 | 4 days | |
| 2 | Fem | 43 | 4 | 1 | LES | Joint, renal, immune | 14 | 0 | 4 days |
| 3 | Fem | 30 | 4 | 5 | LES | Skin, serositis, hematologic, joint, renal, immune | 54 | 7 | 55 days+tracheotomy |
| 4 | Fem | 18 | 3 | 1 | LES | Joint, hematologic, immune | 11 | 0 | No AMV |
| 5 | Male | 32 | 4 | 1 | PAM | Pulmonary, renal | 1 | 10 days | |
| 6 | Fem | 28 | 2 | 1 | LES | Renal, skin w/vasculitis | 13 | 2 | No AMV |
| 7 | Fem | 20 | 1 | 1 | LES | Joint, renal, skin w/vasculitis | 32 | 2 | No AMV |
| 8 | Fem | 30 | 4 | 2 | LES | Serositis | 6 | 0 | 3 days |
| 9 | Fem | 21 | 2 | 1 | PAM | Renal and pulmonary | 2 | No AMV | |
| 10 | Male | 41 | 10 | 1 | LES | Serositis, renal | 13 | 0 | No AMV |
AMV, automated mechanical ventilation; SNP, peripheral nervous system; URA, upper respiratory airway; LRA, lower respiratory airway.
Of the 10 patients, 8 did not have a diagnosis of SLE or systemic vasculitis at the time of the PH and only presented constitutional symptoms in the days before it. One patient had two events of PH, with an interval between the two events of 25 days, the first event was treated with 1g of methylprednisolone every 24h for 3 doses and the second event required AMV for 3 days, accompanied again by 3 pulses of methylprednisolone 1g every 24h. Another patient had 5 events of PH in the period between April 6 and May 12, 2004; during the first event received 7 pulses of methylprednisolone of 1g each and did not require AMV. During the second event (15 days after the first), AMV was required, and the other 3 events also required ventilation as well as management in intensive care, where the patient presented generalized tonic–clonic seizures and bilateral occipital infarction documented by CT. This patient received: methylprednisolone, IV cyclophosphamide, IV immunoglobulin and plasmapheresis for severe SLE activity, and with this the bleeding was controlled. The remaining 8 patients had just one event of PH. The mean SLEDAI score was 20.8 for SLE patients, and in patients with PAM, the FFS was 2 and patients with GP (Wegener) had a BVAS of 36 points (all indices measured at the time of the PH). Of the 7 patients with SLE, only 3 had a higher SLICC score at the time of the study, 2 with a score of 2 and one with a score of 7 (patient 3) with a mean of 2.25 (Table 2).
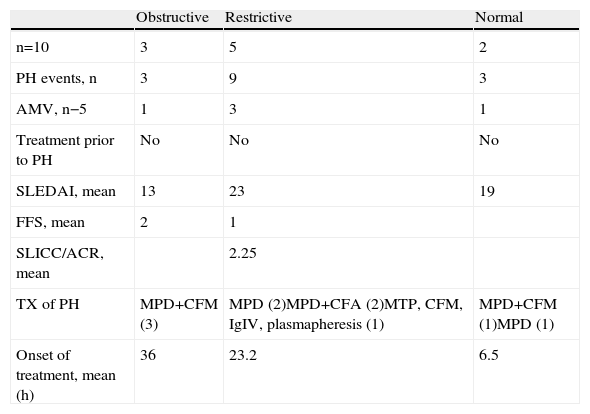
Patterns of Respiratory Mechanics.
| Obstructive | Restrictive | Normal | |
| n=10 | 3 | 5 | 2 |
| PH events, n | 3 | 9 | 3 |
| AMV, n−5 | 1 | 3 | 1 |
| Treatment prior to PH | No | No | No |
| SLEDAI, mean | 13 | 23 | 19 |
| FFS, mean | 2 | 1 | |
| SLICC/ACR, mean | 2.25 | ||
| TX of PH | MPD+CFM (3) | MPD (2)MPD+CFA (2)MTP, CFM, IgIV, plasmapheresis (1) | MPD+CFM (1)MPD (1) |
| Onset of treatment, mean (h) | 36 | 23.2 | 6.5 |
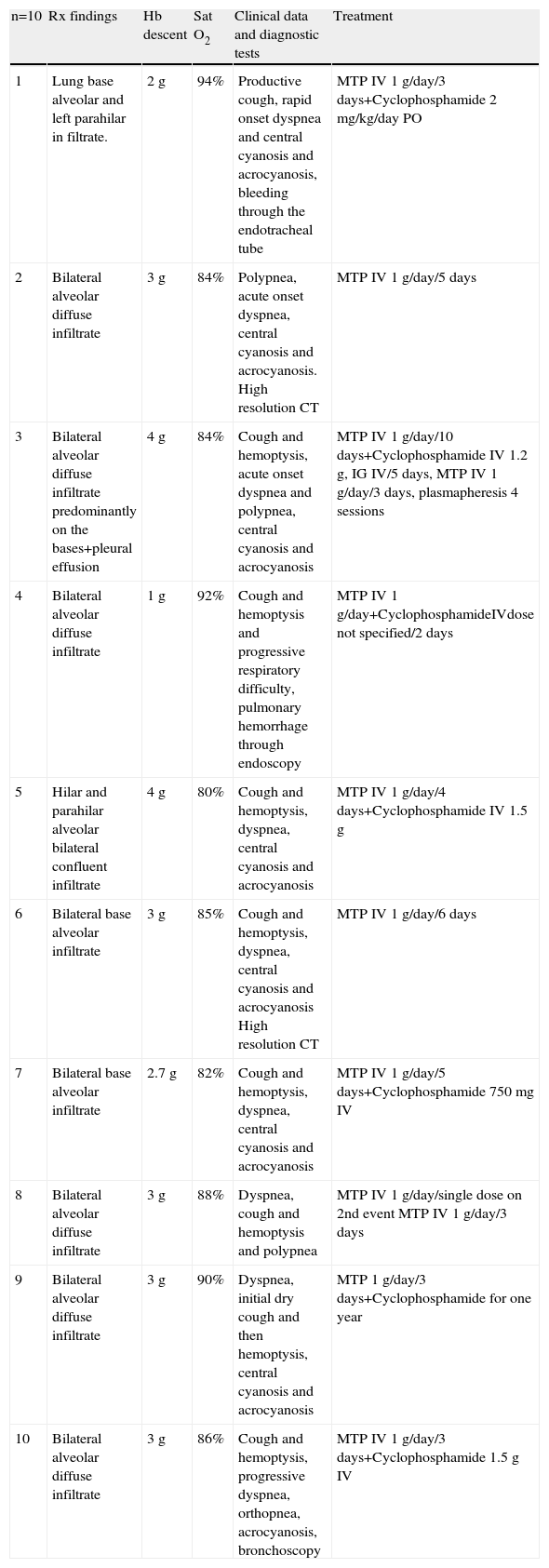
Eight patients presented pulmonary–renal syndrome, including 5 with SLE and 3 with systemic vasculitis (Table 1). Of the 10 patients, 3 were only treated during the acute phase with methylprednisolone (3 with SLE), one patient received methylprednisolone, cyclophosphamide IV, IV immunoglobulin and plasmapheresis, and the remaining 6 received methylprednisolone and cyclophosphamide (Table 3). All patients received antibiotics and we only documented 2 infections, one patient had Candida albicans (in the urinary tract) and Streptococcus spp. (Lung) and one patient had pneumonia associated to prolonged intubation, in whom Calcoaceticus baumanili and Acinetobacter were isolated.
Characteristics at the Moment of the Hemorrhage and Treatment.
| n=10 | Rx findings | Hb descent | Sat O2 | Clinical data and diagnostic tests | Treatment |
| 1 | Lung base alveolar and left parahilar in filtrate. | 2g | 94% | Productive cough, rapid onset dyspnea and central cyanosis and acrocyanosis, bleeding through the endotracheal tube | MTP IV 1g/day/3 days+Cyclophosphamide 2 mg/kg/day PO |
| 2 | Bilateral alveolar diffuse infiltrate | 3g | 84% | Polypnea, acute onset dyspnea, central cyanosis and acrocyanosis. High resolution CT | MTP IV 1g/day/5 days |
| 3 | Bilateral alveolar diffuse infiltrate predominantly on the bases+pleural effusion | 4g | 84% | Cough and hemoptysis, acute onset dyspnea and polypnea, central cyanosis and acrocyanosis | MTP IV 1g/day/10 days+Cyclophosphamide IV 1.2g, IG IV/5 days, MTP IV 1g/day/3 days, plasmapheresis 4 sessions |
| 4 | Bilateral alveolar diffuse infiltrate | 1g | 92% | Cough and hemoptysis and progressive respiratory difficulty, pulmonary hemorrhage through endoscopy | MTP IV 1g/day+CyclophosphamideIVdose not specified/2 days |
| 5 | Hilar and parahilar alveolar bilateral confluent infiltrate | 4g | 80% | Cough and hemoptysis, dyspnea, central cyanosis and acrocyanosis | MTP IV 1g/day/4 days+Cyclophosphamide IV 1.5g |
| 6 | Bilateral base alveolar infiltrate | 3g | 85% | Cough and hemoptysis, dyspnea, central cyanosis and acrocyanosis High resolution CT | MTP IV 1g/day/6 days |
| 7 | Bilateral base alveolar infiltrate | 2.7g | 82% | Cough and hemoptysis, dyspnea, central cyanosis and acrocyanosis | MTP IV 1g/day/5 days+Cyclophosphamide 750mg IV |
| 8 | Bilateral alveolar diffuse infiltrate | 3g | 88% | Dyspnea, cough and hemoptysis and polypnea | MTP IV 1g/day/single dose on 2nd event MTP IV 1g/day/3 days |
| 9 | Bilateral alveolar diffuse infiltrate | 3g | 90% | Dyspnea, initial dry cough and then hemoptysis, central cyanosis and acrocyanosis | MTP 1g/day/3 days+Cyclophosphamide for one year |
| 10 | Bilateral alveolar diffuse infiltrate | 3g | 86% | Cough and hemoptysis, progressive dyspnea, orthopnea, acrocyanosis, bronchoscopy | MTP IV 1g/day/3 days+Cyclophosphamide 1.5g IV |
Hb, hemoglobin; Rx, X-rays; Sat O2, oxygen saturation.
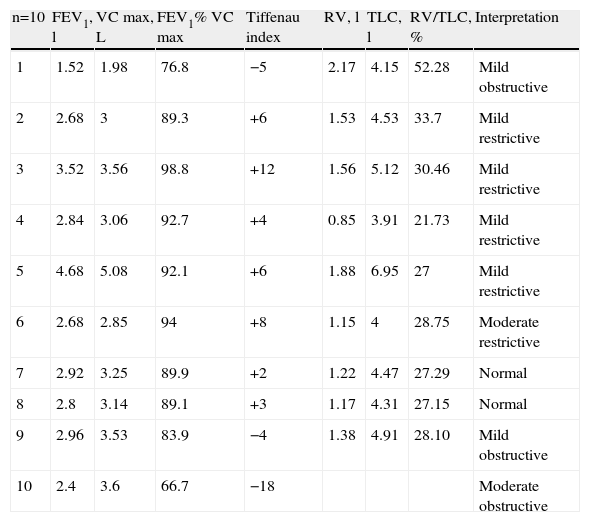
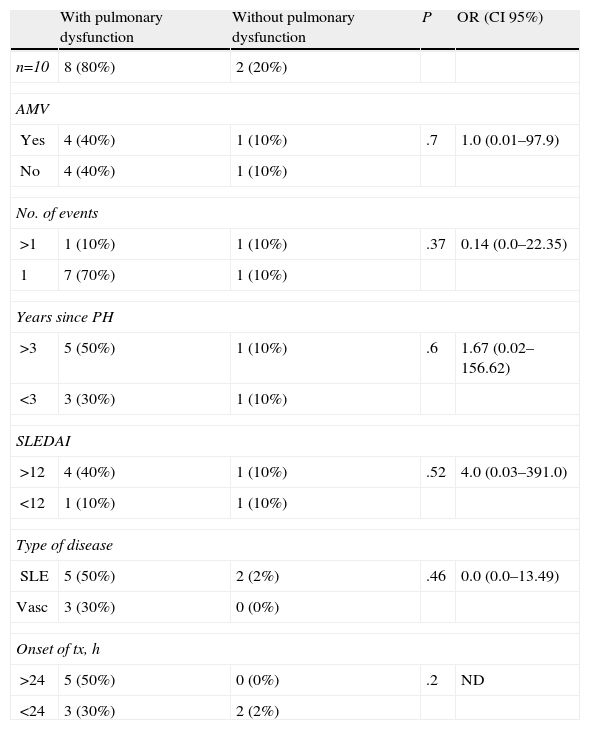
7 of the 10 patients presented the diagnostic triad of PH, but in 3 patients the decrease in hemoglobin was less than 3g. The characteristics at the time of the hemorrhage and treatment given are shown in Table 3. 10 patients underwent respiratory function tests (PFTs), 9 plethysmography and one spirometry. The results are shown in Table 4. We observed an obstructive respiratory pattern in 3 patients, a restrictive pattern in 5 patients and a normal pattern in the remaining 2. According to the Tiffeneau index, 8 patients had chronic respiratory dysfunction, 3 of whom had an obstructive pattern, 2 mild and one moderate. Of the 5 patients who had a restrictive pattern in 4 it was mild and in one moderate. Only one patient had mild hypoxemia and an obstructive pattern (Table 2). It was noted that the two patients who had normal respiratory pattern mechanics were treated at an earlier stage; however, after performing the bivariate analysis there was no statistically significant difference. The same happened with the time evolution of PH, since according to the results, patients with a longer history showed higher respiratory dysfunction when compared with other patients, something not supported by the bivariate analysis. When analyzing the remaining variables to test the chi-square and Fisher's exact test, no statistically significant differences were observed (Table 5).
Respiratory Function Tests: Capacities, Volumes and Tiffenau Index.
| n=10 | FEV1, l | VC max, L | FEV1% VC max | Tiffenau index | RV, l | TLC, l | RV/TLC, % | Interpretation |
| 1 | 1.52 | 1.98 | 76.8 | −5 | 2.17 | 4.15 | 52.28 | Mild obstructive |
| 2 | 2.68 | 3 | 89.3 | +6 | 1.53 | 4.53 | 33.7 | Mild restrictive |
| 3 | 3.52 | 3.56 | 98.8 | +12 | 1.56 | 5.12 | 30.46 | Mild restrictive |
| 4 | 2.84 | 3.06 | 92.7 | +4 | 0.85 | 3.91 | 21.73 | Mild restrictive |
| 5 | 4.68 | 5.08 | 92.1 | +6 | 1.88 | 6.95 | 27 | Mild restrictive |
| 6 | 2.68 | 2.85 | 94 | +8 | 1.15 | 4 | 28.75 | Moderate restrictive |
| 7 | 2.92 | 3.25 | 89.9 | +2 | 1.22 | 4.47 | 27.29 | Normal |
| 8 | 2.8 | 3.14 | 89.1 | +3 | 1.17 | 4.31 | 27.15 | Normal |
| 9 | 2.96 | 3.53 | 83.9 | −4 | 1.38 | 4.91 | 28.10 | Mild obstructive |
| 10 | 2.4 | 3.6 | 66.7 | −18 | Moderate obstructive |
FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; RV, reserve volume; TLC, total lung capacity; VC max, maximum vital capacity.
Association Between the Included Variables and Pulmonary Hemorrhage.
| With pulmonary dysfunction | Without pulmonary dysfunction | P | OR (CI 95%) | |
| n=10 | 8 (80%) | 2 (20%) | ||
| AMV | ||||
| Yes | 4 (40%) | 1 (10%) | .7 | 1.0 (0.01–97.9) |
| No | 4 (40%) | 1 (10%) | ||
| No. of events | ||||
| >1 | 1 (10%) | 1 (10%) | .37 | 0.14 (0.0–22.35) |
| 1 | 7 (70%) | 1 (10%) | ||
| Years since PH | ||||
| >3 | 5 (50%) | 1 (10%) | .6 | 1.67 (0.02–156.62) |
| <3 | 3 (30%) | 1 (10%) | ||
| SLEDAI | ||||
| >12 | 4 (40%) | 1 (10%) | .52 | 4.0 (0.03–391.0) |
| <12 | 1 (10%) | 1 (10%) | ||
| Type of disease | ||||
| SLE | 5 (50%) | 2 (2%) | .46 | 0.0 (0.0–13.49) |
| Vasc | 3 (30%) | 0 (0%) | ||
| Onset of tx, h | ||||
| >24 | 5 (50%) | 0 (0%) | .2 | ND |
| <24 | 3 (30%) | 2 (2%) | ||
PH is a rare complication of autoimmune diseases, but is associated with high morbidity and mortality. PH series published in the literature are descriptive and include small numbers of patients; one of the largest, Barile et al., included 34 patients with PH and SLE.11 PH is presented clinically with a very heterogeneous pattern, from a chronic form to a catastrophic event of acute massive hemoptysis. The clinical manifestations of PH include a decrease of hemoglobin, acute respiratory failure, X-ray infiltrates and hemoptysis, although the latter may be absent, as demonstrated by Abud-Mendoza et al.10 in their series of 12 cases published in 1985, a finding corroborated by Barile et al. in 1997, in a series that showed absence of hemoptysis in 41% of the cases.11 These studies agree that the triad composed of acute respiratory distress, a decline in hemoglobin >3g/dl and radiological alveolar infiltrates in at least 3 quarters of the lungs are necessary to reach the diagnosis of PH, although in early stages the decrease in hemoglobin may be minor and infiltration covers less than 3 quarters of the lung parenchyma. In these cases, the diagnosis is established by bronchoscopy or a high resolution CT. In our study, 7 of the 10 patients showed the triad, but 3 of them had a descent in hemoglobin of less than 3g. In the majority of patients with secondary PH, this was the initial manifestation of the disease; this has been reported in 12%–20% of cases. In the case of the WG (Wegener), PH appears in 42% of cases and in PAM in 29% of the cases.24
In the present study, we found that 5 of the patients with SLE and 3 patients with systemic vasculitis did not have an established diagnosis and had only presented constitutional symptoms prior to PH. Only 2 patients had an established diagnosis of disease 2 months prior to PH and were not taking immunosuppressives or glucocorticoid.
It has been described that 75%–93% of lupus cases of PH occur as a lung–kidney syndrome,10,11 which agrees with our study. The physiopathogeny of PH is not fully understood or constitutes a uniform process for all entities grouped under this nomenclature. Although it is frequent to find it associated with capillaritis, this association is not present in 100% of cases. The presence of capillaritis is a pathological finding that could point toward systemic vasculitis16 such as SLE, GP or PAM, among others. In the case of diseases in which capillaritisis histologically seen, it seems that immune complexes deposits plays a very important role in the development of these conditions, with complement activation, as well as release of vasoactive amines and chemotactic factors.
The presence of circulating or base-membrane deposited immune complexes favors the development of vascular inflammation,25 but their absence does not mean that they do not participate in the pathogenesis of PH, since they can be removed from both the circulation and tissues by granulocytes within hours of their generation.26
Neutrophil cytoplasmic antibodies are involved in the pathogenesis of GP and PAM systemic vasculitis, and are important in the activation of circulating monocytes and neutrophils, and cross-react with antigens present on the endothelial surface. In general, the activation of neutrophils triggers free oxygen radical and lysosomal enzyme release, causing endothelial injury.
Recurrent episodes of PH may lead to interstitial fibrosis and a restrictive lung pattern, as demonstrated in lung function tests (ventilation-perfusion or spirometry) with a reduction in TLC and FVC and preservation of the FEV1/FVC ratio; obstructive changes may also be found, demonstrated as an increase in TLC and a reduction in the FEV1/CVF ratio. As mentioned, we found respiratory function alterations in 8 of our patients, 3 with an obstructive pattern and 5 with a restrictive one.
No association was found between disease activity (SLE or vasculitis), the use of AMV, time since onset of treatment, years since PH or number of PH events with the development of chronic respiratory dysfunction, probably due to the sample size, with only 10 patients. This is a study limitation.
In conclusion, we found chronic respiratory distress in a high percentage of patients in whom the probable mechanism of action was acute inflammation and a Th1 cytokine patter and then chronic damage with a Th2 cytokine pattern. It is also likely that long-term immunosuppressive treatment is needed to solve the acute PH episode. This is the only study that has measured lung function in PH patients with SLE and primary systemic vasculitis. Controlled prospective studies with a greater number of subjects are needed to corroborate these findings.
Ethical ResponsibilitiesProtection of persons and animalsThe authors declare that for this research no experiments were performed on humans or animals.
Data confidentialityThe authors declare that they have followed their workplace guidelines on the publication of patient data and all included patients have received sufficient information and have given their informed consent in writing in order to participate in the study.
Right to privacy and informed consentThe authors have obtained the informed consent of patients and/or subjects in this paper. This document is in the possession of the corresponding author.
Conflicts of InterestThe authors have no disclosures to make.
Please cite this article as: Pérez Aceves E, Pérez Cristóbal M, Espinola Reyna GA, Ariza Andraca R, Xibille Fridmann D, Barile Fabris LA. Disfunción respiratoria crónica por hemorragia alveolar difusa en pacientes con lupus eritematoso sistémico y vasculitis primaria. Reumatol Clin. 2013;9:263–268.