To determine to neutrophil-to-lymphocyte ratio (NLR) in granulomatosis with polyangiitis (GPA) patients and to study its relation to disease manifestations and activity.
MethodsThe study included 44 GPA patients and 44 matched age and sex controls. Full history taking, thorough clinical examination with more attention to ocular examination, laboratory and radiological investigations were considered. Disease activity was assessed using the Birmingham Vasculitis Activity Score (BVAS).
ResultsThe patients (21 males and 23 females) had a mean age of 45.66±7.24 years, disease duration 6.8±3.6 years and BVAS 50.1±14.3. All patients had a positive cytoplasmic anti-neutrophil cytoplasmic antibody (c-ANCA) while only 5 had a positive p-ANCA. The NLR was significantly increased in the GPA patients (5.1±2.4) compared to the control (1.5±0.8) (P<.0001). Ten patients with uveitis had a significantly higher NLR (6.5±1.9) compared to those without (4.7±2.4) (0.03) while those with proptosis (n=10), cutaneous manifestations (n=17) or ischemic heart disease (n=9) had a significantly lower NLR than those without (P=.0001, P=.017 and P=.046 respectively). The NLR did not significantly correlate with any of the patients’ characteristics. The NLR inversely yet insignificantly correlated with the disease activity (r=−0.02, P=.93).
ConclusionThe NLR may have a significant role in the pathogenesis of GPA, the development of uveitis or proptosis, cutaneous manifestations and ischemic heart disease. NLR may serve as a future potential companion to c-ANCA positivity in diagnosing and evaluating GPA and may play a role in the tissue-specific and clinical characteristics.
Determinar el ratio neutrófilos/linfocitos (RNL) en pacientes con granulomatosis con poliangeítis (GP), y estudiar su relación con las manifestaciones y actividad de la enfermedad.
MétodosEl estudio incluyó a 44 pacientes con GP, y 44 controles pareados por edad y sexo. Se consideraron la historia clínica completa, la exploración minuciosa con especial atención al examen ocular, así como las pruebas de laboratorio y radiológicas. La actividad de la enfermedad se evaluó utilizando la clasificación Birmingham Vasculitis Activity Score (BVAS).
ResultadosLos pacientes (21 varones y 23 mujeres) tenían una edad media de 45,66±7,24 años, duración de la enfermedad de 6,8±3,6 años, y BVAS 50,1±14,3. Todos los pacientes tenían anticuerpos anticitoplasma de anti-neutrófilos positivos (c-ANCA), y únicamente 5 de ellos tenían p-ANCA positivo. El RNL se vio significativamente incrementado en los pacientes de GP (5,1±2,4) en comparación con el grupo control (1,5±0,8) (p<0,0001). En 10 pacientes con uveítis se observó un RNL significativamente superior (6,5±1,9) en comparación con aquellos sin uveítis (4,7±2,4) (0,03), mientras que en aquellos con proptosis (n=10), manifestaciones cutáneas (n=17) o cardiopatía isquémica (n=9) se observó un RNL significativamente inferior al de aquellos sin dichas manifestaciones (p=0,0001; p=0,017 y p=0,046, respectivamente). El RNL no guardó una correlación significativa con ninguna de las características de los pacientes. Sin embargo, el RNL guardó una correlación no significativa con la actividad de la enfermedad (r=−0,02; p=0,93).
ConclusiónEl RNL puede desempeñar un papel significativo en la patogenia de la GP, el desarrollo de uveítis o proptosis, manifestaciones cutáneas y cardiopatía isquémica. El RNL puede servir como futuro complemento potencial de la positividad de c-ANCA a la hora de diagnosticar y evaluar la GP, y jugar un papel con relación a sus características tisulares específicas y clínicas.
Granulomatosis with polyangiitis (GPA) is an infrequent multisystemic necrotizing vasculitis, typically involving the respiratory systems and kidneys and usually runs a relapsing course.1 GPA is an antineutrophil cytoplasmic antibody (ANCA) associated vasculitis (AAV). Necrotizing granulomatous inflammation mainly involving the small-medium vessels leads to major complications up to mortality with increased resistance to medications.2 In this disease, proteinase-3 (PR3), produced by neutrophils, is targeted by ANCA.3
Neutrophils are important in the defense against pathogens, in modulating inflammation and in fine-tuning immune responses.4 They are actively involved in systemic and local inflammatory responses by releasing pro-inflammatory elements5 thus playing a role in many rheumatic diseases, yet the underlying molecular mechanisms are not fully revealed.4 Neutrophils may be identified in the lesions of virtually all kinds of vasculitis with variable mechanisms of accumulation. In AAV diseases, partial neutrophil degranulation results in exposure of ANCA antigens (myeloperoxidase ‘MPO’ and PR3).6 Neutrophils play an immunostimmulant role in the pathogenesis of GPA.7 A wide range of antigens are recognized by lymphocytes and the immune system contains subpopulations specialized in recognizing conserved structures. Given the numerous steps in the development of lymphocytes, it is expected that many causes may lead to their immunodeficiency.8 Increased frequency of Th-cells in ANCA-positive GPA patients throw light on a possible role of these cells in the immunopathogenesis of the disease.9 Of the ANCA-associated vasculitides, GPA best demonstrates the theory that autoimmunity can develop against one specific neutrophil protein, namely, PR3. The unique features of PR3 are primary contributors to the systemic inflammation and to the immune dysregulation involved in GPA.4
The neutrophil-to-lymphocyte ratio (NLR) is a useful marker that predicts not only disease progression, but also mortality in different inflammatory diseases1 making it noticeable as a new biomarker of inflammation.5
The Birmingham Vasculitis Activity Score (BVAS) is a regular activity score used in GPA. Even though anti-PR3 antibodies are specific for GPA, the role of its level or of any other laboratory test as an activity marker has not been established.10–12
The aim of the present work was to determine to NLR in GPA patients and to study its relation to disease manifestations and activity.
Patients and MethodsThe study included 44 GPA patients recruited from Ain Shams and Alexandria University hospitals diagnosed according to the 1990 American College of Rheumatology criteria for GPA.13 Forty-four matched age and sex controls were also included. The study is in accordance to the 1995 Helsinki declaration and approved by the ethical committee of Ain Shams and Alexandria Universities’ Hospitals. Informed consent was obtained from all patients. Patients with diabetes mellitus, chronic renal failure, chronic obstructive pulmonary disease, coronary artery disease, malignancy, hematologic abnormality, acute or chronic infection, with a granulomatous chronic disease, pregnant or post-partum were excluded.
Full history taking, thorough clinical examination with more attention to ocular manifestations and slit lamp examination, laboratory and radiological investigations were undertaken for all patients and their medications received were also reported. The complete blood count (CBC), erythrocyte sedimentation rate (ESR), liver and kidney function test, lipid profile, complement (C3 and C4) were measured. The rheumatoid factor (RF) and anti-nuclear antibody (ANA) were evaluated. The ANCA (anti-PR3 and anti-MPO) were determined by combining the results of indirect immunofluorescence tests for cytoplasmic (c-ANCA) and perinuclear (p-ANCA) patterns with those of ELISA directed to measure antigen. From the CBC differential, the absolute neutrophil count was divided by the absolute lymphocyte count to calculate the NLR. The normal NLR values in adult, non-geriatric, population in good health is reported to range between 0.78 and 3.53.14 Disease activity was assessed using the BVAS.15
ResultsThe study included 44 GPA patients (21 males and 23 females) with a mean age of 45.66±7.24 years and 44 matched control for age (43.07±10.15 years; P=.17) and gender (P=.83). The disease duration of the patients was 6.8±3.6 years and their mean body mass index was 25.4±2.4 and 24 (54.5%) were hypertensive. None of the patients had any associated inflammatory conditions. The clinical and laboratory findings as well as the BVAS of the GPA patients are presented in Table 1. Ten patients had significant proptosis, 38 scleritis/episcleritis, 32 conjunctivitis/blepharitis/keratitis, 10 uveitis and 6 retinal changes. Nasal manifestations were present in 29 patients, sinusitis in 33, subglottic stenosis in 2 and hearing impairment in 11. Thirty-six patients had a wheezy chest, 12 had pulmonary nodules and cavities, 5 had pleural effusion, 8 pulmonary infiltrates and 4 massive hemoptysis. All the patients were receiving steroids, 7 received methotrexate, 35 cyclophosphamide, 38 azathioprine and 3 mycophenolate mofetil. None of the patients received a biologic therapy.
Clinical and Laboratory Characteristics as well as Disease Activity of the Granulomatosis With Polyangiitis Patients.
| Parameter | GPA patients (n=44) | |
|---|---|---|
| Clinical manifestations | Fever (>38°C) | 41 (93.2) |
| Myalgias | 42 (95.5) | |
| Weight loss | 38 (86.4) | |
| Cutaneous | 17 (38.6) | |
| Oral ulcers | 9 (20.5) | |
| Arthralgia/arthritis | 43 (97.7) | |
| Eye | 44 (100) | |
| ENT | 42 (95.5) | |
| Pulmonary | 42 (95.5) | |
| Cardiac | 24 (54.5) | |
| Gastrointestinal | 14 (31.8) | |
| Renal | 27 (61.4) | |
| Neurological | 27 (61.4) | |
| Laboratory investigations | Hemoglobin (g/dl) | 10.7±1.5 |
| WBC (×109/l) | 11.4±2.7 | |
| Neutrophils (×109/l) | 8.7±2.1 | |
| Lymphocytes (×109/l) | 1.9±0.7 | |
| NLR | 5.1±2.4 | |
| Esinophils (%) | 2.3±0.9 | |
| Platelets (×103/mm3) | 286.7±73.2 | |
| ESR (mm/1st h) | 48.7±19.9 | |
| FBS (mg/dl) | 97.7±29.4 | |
| AST (IU/ml) | 21.1±4.1 | |
| ALT (IU/ml) | 23.6±4.8 | |
| ALP (U/L) | 115.6±31.1 | |
| Albumin (mg/dl) | 4.03±0.7 | |
| Creatinine (mg/dl) | 2.04±1.7 | |
| Urea (mg/dl) | 38.2±18.3 | |
| SUA (mg/dl) | 6.5±9.03 | |
| Cholesterol (mg/dl) | 180.9±48.3 | |
| Triglycerides (mg/dl) | 164.7±47.7 | |
| c-ANCA positivity | 44 (100) | |
| p-ANCA positivity | 5 (11.4) | |
| ANA positivity | 20 (45.5) | |
| RF positivity | 25 (56.8) | |
| C3 (mg/dl) | 1.29±0.3 | |
| C4 (mg/dl) | 0.28±0.1 | |
| BVAS | 50.1±14.3 | |
GPA: granulomatosis with polyangiitis, ENT: ear, nose and throat, WBC: white blood cells, NLR: neutrophil-to-lymphocyte ratio, ESR: erythrocyte sedimentation rate, FBS: fasting blood sugar, AST: aspartate transaminase, ALT: alanine transaminase, ALP: alkaline phosphatase, SUA: serum uric acid, ANCA: antineutrophil cytoplasmic antibody-cytoplasmic and perinuclear, ANA; anti-nuclear antibody, RF: rheumatoid factor, C: complement, BVAS: Birmingham Vasculitis Activity Score.
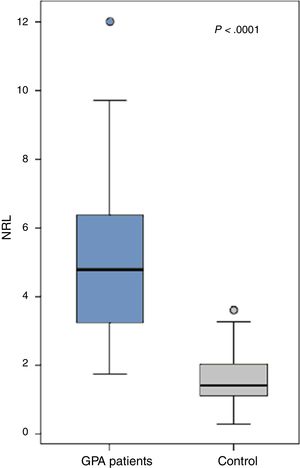
The NLR was significantly increased in the GPA patients (5.1±2.4) compared to the control (1.5±0.8) (P<.0001) (Fig. 1). The levels were comparable between GPA male (5.2±2.4) and females (5±2.4) (P=.69). Comparing the NLR according to the presence and absence of the clinical manifestations, autoantibodies positivity and medications received are shown in Table 2.
The Neutrophil-to-lymphocyte Ratio According to the Presence and Absence of Clinical Manifestations, Autoantibodies Positivity and Medications Received.
| Clinical manifestationmean±SD | GPA patients (n=44) | ||
|---|---|---|---|
| Presence | Absence | P | |
| Cutaneous (n=17) | 4.1±1.8 | 5.7±2.6 | (.017) |
| Oral ulcers (n=9) | 4.7±2.4 | 5.2±2.4 | (.61) |
| Proptosis (n=10) | 3.5±1.2 | 5.6±2.5 | (.0001) |
| Scleritis (n=38) | 5.3±2.5 | 4.1±2.01 | (.25) |
| Conjunctivitis (n=32) | 5.2±2.4 | 4.8±2.5 | (.68) |
| Uveitis (n=10) | 6.5±1.9 | 4.7±2.4 | (.03) |
| Retinal changes (n=6) | 6.7±3.7 | 4.9±2.1 | (.3) |
| Nasal (n=29) | 4.9±2.4 | 5.4±2.4 | (.57) |
| Sinusitis (n=33) | 4.9±2.2 | 5.6±2.9 | (.51) |
| Hearing impairment (n=11) | 4.6±2.3 | 5.3±2.4 | (.42) |
| Nodules and cavities (n=12) | 4.3±1.7 | 5.4±2.6 | (.12) |
| Pleural effusion (n=5) | 4.7±2.4 | 5.2±2.4 | (.69) |
| Infiltrates (n=8) | 5.1±2.7 | 5.1±2.4 | (.95) |
| Cardiac (n=24) | 4.9±2.2 | 5.4±2.7 | (.51) |
| Ischemic heart disease (n=9) | 4.1±1.4 | 5.4±2.6 | (.046) |
| Gastrointestinal (n=14) | 5.4±2.5 | 4.9±2.4 | (.56) |
| Renal (n=27) | 5.4±2.4 | 4.6±2.4 | (.28) |
| Neurological (n=27) | 5±2.4 | 5.3±2.5 | (.74) |
| p-ANCA (n=5) | 4.8±1.9 | 5.1±2.5 | (.71) |
| ANA positivity (n=20) | 5.1±2.6 | 5.1±2.3 | (.95) |
| RF positivity (n=25) | 5±2.2 | 5.2±2.7 | (.8) |
| MTX (n=7) | 4.4±2.3 | 5.2±2.4 | (.44) |
| CYC (n=35) | 5.4±2.5 | 4±1.7 | (.06) |
| AZA (n=38) | 5±2.1 | 5.5±4.1 | (.8) |
GPA: granulomatosis with polyangiitis, p-ANCA: perinuclear antineutrophil cytoplasmic antibody, ANA; anti-nuclear antibody, RF: rheumatoid factor, MTX: methotrexate, CYC: cyclophosphamide, AZA: azathioprine.
The NLR did not significantly correlate with any of the patients’ characteristics. The neutrophilic count, lymphocytic count and NLR inversely yet insignificantly correlated with the disease activity (r=−0.12, P=.42; r=−0.14, P=.36; r=−0.02, P=.93).
DiscussionGranulomatosis with polyangiitis is a vasculitis with potential morbidity and mortality.16 In GPA, neutrophils play a pivotal role in its pathology.17 c-ANCAs are present in 90% of GPA patients. As the humoral immunity is predominantly directed against neutrophilic antigens, it is apparent that neutrophils play a critical role in GPA both as target and effector cells.18 The pathophysiological importance of PR3-ANCA is not yet understood and the pathogenic pathways leading to granuloma formation are not elucidated.19
In the present study, the NLR significantly increased in the GPA patients compared to the control which was in agreement to the results of another recent study.1 It is unknown why ANCA develops, yet it plays a critical role in the disease pathogenesis by activating neutrophils. The accretion of apoptotic neutrophils progresses into secondary necrosis leading to tissue damage and autoantibody formation.20 Neutrophilia and neutrophil chemotaxis have been reported to persist even in clinical remission of GPA cases.21 Inflammatory cells as neutrophils are implicated in the pathophysiology of AAV.22
Ocular involvement frequently occurs in GPA patients23 and may lead to orbital bone destruction and loss of vision.24 Ocular features of GPA include scleritis, which can be necrotizing, bilateral and can pose high threat to the globe.25 On considering the presence and absence of clinical manifestations, there was obvious NLR imbalance being higher in those with uveitis and lower in those with proptosis, cutaneous manifestations and ischemic heart disease. Neutrophilic infiltration of the ocular tissues, together with vasculitis and necrosis, are all associated with a clinical diagnosis of GPA and may help in confirming an early orbital disease especially in patients with a negative ANCA.25 Similar to another rheumatic disease, Behçet's disease (BD), NLR were increased in patients with uveitis.26
A decrease in lymphocytes and increased number of neutrophils in AAV patients was reported. Furthermore, ANCA may play a role by decreasing phagocytic ability.20 Increased TH17 lymphocytes during acute conditions and a defect in T regulatory (Treg) cells in chronic phases have been identified in GPA.22 In GPA, Th17 lymphocytes are pathogenic and Treg cells suppressors of inflammation. These two subsets are of value in the follow-up of patients once immunosuppressives are initiated.27 In GPA, expansion of Treg and Th2 lymphocytes in parallel to increased Th17 response is a characteristic feature of sustained remission. In contrast, Treg cells are markedly decreased in disease flare.28 The role of B-lymphocytes in the pathogenesis of AAV is now well documented by the effectiveness of rituximab in the treatment of this condition.22 B-cell survival factors in the mucosa in GPA are responsible for chronically activated B-cells.29 It could thus seem useful to consider NLR as a possible potential marker of treatment response in a longitudinal study.
In GPA patients, there is an expansion of another subset of lymphocytes, effector memory T-cells including T-helper 1 (Th1) and Th17 cells. These T-cells play an important role in tissue damage and disease progression in AAV.30 Other lymphocytes, NK cells, were not detectable in GPA granulomas and inversely correlated with disease activity.12
In the present work, the NLR tended to be higher in those on CYC. It has been reported that immunosuppressives and steroids may shift lymphocyte proportions. Patients with active GPA typically receive potent induction therapy which is accompanied with lower NK counts. The less potent maintenance therapy in inactive GPA was indirectly associated with elevated cell counts. Increased NK cell proportions are more likely a correlate of clinical amelioration rather than an alteration of medications.12
In GPA, tissue-specific characteristics of microvascular endothelial cells may shape their interaction with NK cells and determine their susceptibility to immunological attack and may have a key role in their recruitment and migration to inflammatory sites of vasculitis.31 It has been suggested that blood NLR may be used for predicting vasculitis, especially cutaneous forms with systemic involvement.32 Also in another vasculitic syndrome, Kawasaki disease, patients with aneurysms, but not patients with dilatation, had higher NLRs than patients without coronary artery abnormalities.33
In the present study the NLR did not significantly correlate with the disease activity. It has been found that platelet-neutrophil aggregates were considerably associated to the disease activity score (BVAS) in GPA patients.34 In another study, higher NLR at baseline was associated with worse renal outcome in GPA and significantly correlated with BVAS activity scores. GPA patients with a high NLR may require close follow-up for persistent disease activity.1 Moreover, in GPA, a persistent unbalanced expansion of Th17 cells and Th17 subsets seems to be independent of disease activity.35
The cross sectional study design and moderate number of patients are among the limitations of this work. Further longitudinal studies on a larger sample are recommended to validate the present results and postulate a possible diagnostic potential.
In conclusion, NLR may have a significant role in the pathogenesis of GPA, the development of uveitis or proptosis, cutaneous manifestations or ischemic heart disease. Based on the present results and on the previous literature, NLR may serve as a potential companion to c-ANCA positivity in diagnosing and evaluating GPA and may play a role in the tissue-specific and clinical characteristics.
Conflict of InterestNone declared.