Septic arthritis is a medical emergency and crystal-induced arthritis is a risk factor for its development. If both occur simultaneously, crystal-induced arthritis may mask the diagnosis of infection and delay antibiotic therapy.
MethodRetrospective analysis of patients with coexistence of septic and crystal-induced arthritis. We included only patients with isolation of crystals in synovial fluid analysis and positive culture of synovial fluid and/or blood culture.
ResultsA total of 25 patients (17 men and 8 women) with a mean age of 67 years. The most commonly affected joint was the knee. In synovial fluid cytological studies, the most frequently identified crystals were monosodium urate. Risk factors included diabetes and chronic renal failure. The most frequently isolated germs were methicillin-sensitive Staphylococcus aureus (48%), methicillin-resistant S. aureus (12%) and Mycobacterium tuberculosis (12%). In all, 36% of subjects required surgical drainage (excluding those caused by M. tuberculosis). Clinical outcome was favourable in 56%, although intercurrent complications were usual (40%). Mortality was 8%.
ConclusionsCoexistence of septic and crystal-induced arthritis represents a diagnostic challenge and requires a high index of suspicion. Gout was the most prevalent crystal-induced arthritis. S. aureus was the most commonly causative pathogen, with a high rate of methicillin-resistant S. aureus infection. If treated early, the outcome is usually favourable, making synovial fluid microbiological study imperative.
La artritis séptica es una urgencia médica y la artritis microcristalina es un factor de riesgo para su aparición. Si ambas cursan de forma simultánea, la identificación de microcristales puede enmascarar el diagnóstico de la infección y causar un retraso en la instauración del tratamiento antibiótico.
MétodoAnálisis retrospectivo de pacientes con coexistencia de artritis séptica y microcristalina. Se incluye únicamente a los enfermos con aislamiento del germen en líquido articular y/o hemocultivo e identificación de cristales en el líquido articular.
ResultadosSe identificaron un total de 25 pacientes (17 varones y 8 mujeres) con una media de edad de 67 años. La articulación que se afectó con mayor frecuencia fue la rodilla. Los cristales de urato monosódico fueron los que con mayor frecuencia se identificaron en el estudio citológico del líquido sinovial. Los factores de riesgo más frecuentes fueron la diabetes mellitus y la insuficiencia renal crónica. El germen aislado con mayor frecuencia fue el Staphylococcus aureus sensible a meticilina (48%), seguido del Staphylococcus aureus resistente a meticilina (12%) y Mycobacterium tuberculosis (12%). El 36% de los pacientes precisaron desbridamiento quirúrgico (excluyendo los causados por M. tuberculosis). La evolución fue favorable en el 56% de los pacientes, aunque la presencia de complicaciones intercurrentes fue habitual (40%). La mortalidad fue del 8%.
ConclusionesLa coexistencia de artritis séptica y microcristalina representa un reto diagnóstico y requiere un alto índice de sospecha. La artropatía por cristales de urato monosódico es la más prevalente y S. aureus el germen causal más frecuente, con una tasa elevada de infección por S. aureus resistente a meticilina. Si se instaura de forma precoz el tratamiento adecuado, la evolución suele ser favorable, por lo que el estudio microbiológico del líquido sinovial es imperativo.
Septic arthritis is considered a medical emergency due to the rapid anatomical and functional impairment it causes to the affected joint. Arthropathy due to deposition of crystals is a risk factor for its onset. If both occur simultaneously, crystal-induced arthritis can mask a diagnosis of infection, and result in a delay in starting antibiotic treatment. Therefore microbiological study of synovial fluid (Gram staining and cultures) is essential for all episodes of acute arthritis, even if the presence of crystals is detected in the fluid. Although it is not a common situation in clinical practice, the possibility of infection should be considered in all cases of crystal-induced arthritis.
The objective of this paper was to assess the characteristics of patients attended in our hospital with concomitant septic and crystal-induced arthritis. In the Spanish-speaking literature there are 7 case reports,1–7 but no published series. We only found 2 case series in the English-speaking medical literature,8,9 which justifies this study.
Patients and methodWe performed a retrospective analysis of septic and crystal-induced arthritis cases registered in a rheumatology section, in the setting of a university hospital covering a referral population of around 850,000 inhabitants.
An analysis was made of all the infectious arthritis cases (code IVA of the ACR classification)10 gathered in the service registry from 1985 to 2015 (database with coding of diagnoses). For the purposes of the study we chose the cases with germs in the synovial fluid study and/or in the blood cultures. A total of 113 patients were collected, of whom those who had concomitant arthritis due to crystal deposition (code VA) were selected,10 defined by the presence of intracellular crystals in the synovial fluid.
The variables recorded included: age, sex, infection risk factors, sites of joint infection and deposition of microcrystals, fever (axillary temperature above 37.5°C), time until diagnosis (days), Gram staining and synovial fluid cultures, acute phase reactants on diagnosis (erythrocyte sedimentation rate and C-reactive protein), synovial fluid cellularity, cytology study of synovial fluid on polarised light optical microscopy, antibiotic treatment given (administration route and duration), surgical drainage, outcome, and intercurrent complications.
The radiographs or radiological reports relevant to the diagnosis were reviewed where possible.
The method was descriptive, and the results were compared with the principal references from the medical literature.
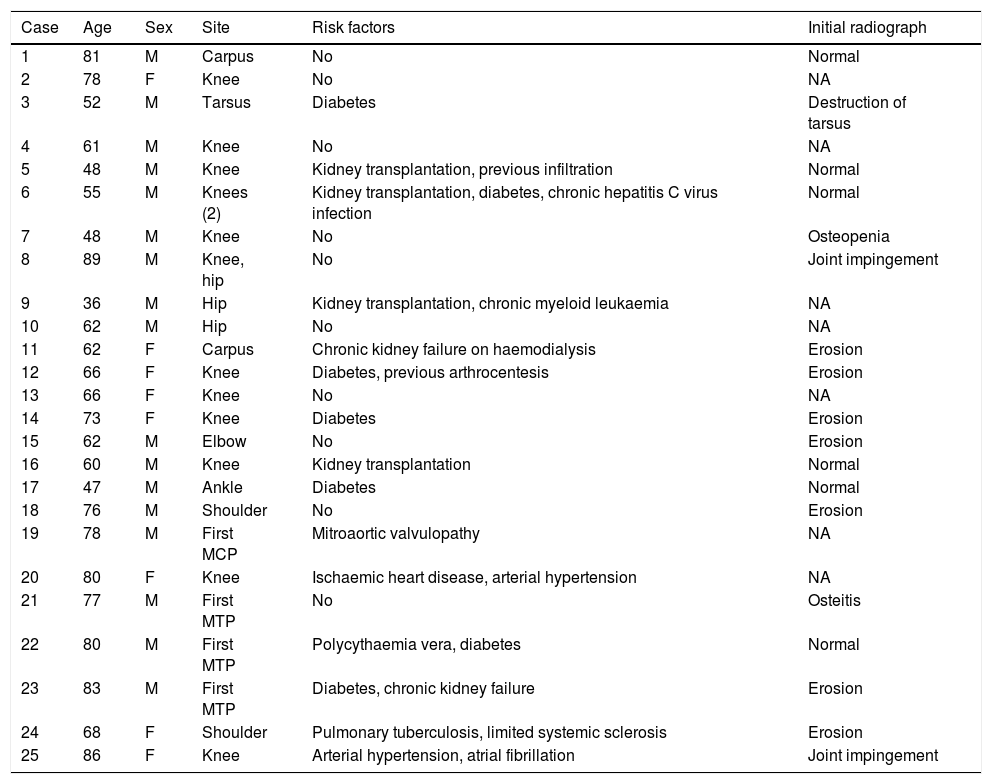
ResultsBetween 1985 and December 2015, 25 patients with concomitant crystal-induced and septic arthritis were registered in the rheumatology section of the University Hospital Germans Trias i Pujol of Badalona. The distribution by sex was 17 males and 8 females, with a mean±standard deviation (interval) of age of 67±14 (36–89) years. The mean±standard deviation (interval) of time between onset of symptoms and diagnosis was 14±13 (1–45) days (data available for 14 patients). The knee was the most frequently affected joint, followed by the foot and hip. In 2 cases various joints were involved at once (Table 1). The general characteristics of the series, as well as the radiological characteristics on diagnosis, are shown in Table 1.
General description of the series and radiological characteristics.
| Case | Age | Sex | Site | Risk factors | Initial radiograph |
|---|---|---|---|---|---|
| 1 | 81 | M | Carpus | No | Normal |
| 2 | 78 | F | Knee | No | NA |
| 3 | 52 | M | Tarsus | Diabetes | Destruction of tarsus |
| 4 | 61 | M | Knee | No | NA |
| 5 | 48 | M | Knee | Kidney transplantation, previous infiltration | Normal |
| 6 | 55 | M | Knees (2) | Kidney transplantation, diabetes, chronic hepatitis C virus infection | Normal |
| 7 | 48 | M | Knee | No | Osteopenia |
| 8 | 89 | M | Knee, hip | No | Joint impingement |
| 9 | 36 | M | Hip | Kidney transplantation, chronic myeloid leukaemia | NA |
| 10 | 62 | M | Hip | No | NA |
| 11 | 62 | F | Carpus | Chronic kidney failure on haemodialysis | Erosion |
| 12 | 66 | F | Knee | Diabetes, previous arthrocentesis | Erosion |
| 13 | 66 | F | Knee | No | NA |
| 14 | 73 | F | Knee | Diabetes | Erosion |
| 15 | 62 | M | Elbow | No | Erosion |
| 16 | 60 | M | Knee | Kidney transplantation | Normal |
| 17 | 47 | M | Ankle | Diabetes | Normal |
| 18 | 76 | M | Shoulder | No | Erosion |
| 19 | 78 | M | First MCP | Mitroaortic valvulopathy | NA |
| 20 | 80 | F | Knee | Ischaemic heart disease, arterial hypertension | NA |
| 21 | 77 | M | First MTP | No | Osteitis |
| 22 | 80 | M | First MTP | Polycythaemia vera, diabetes | Normal |
| 23 | 83 | M | First MTP | Diabetes, chronic kidney failure | Erosion |
| 24 | 68 | F | Shoulder | Pulmonary tuberculosis, limited systemic sclerosis | Erosion |
| 25 | 86 | F | Knee | Arterial hypertension, atrial fibrillation | Joint impingement |
F: female; MCP: metacarpophalangeal; MTP: metatarsophalangeal; NA: not available; M: male.
The most frequent risk factors were: diabetes mellitus (24%), chronic kidney failure (16%), kidney transplantation (16%), arthrocentesis or previous infiltration (8%), and arterial hypertension (8%). Some patients had more than one risk factor, while none were found in 40%.
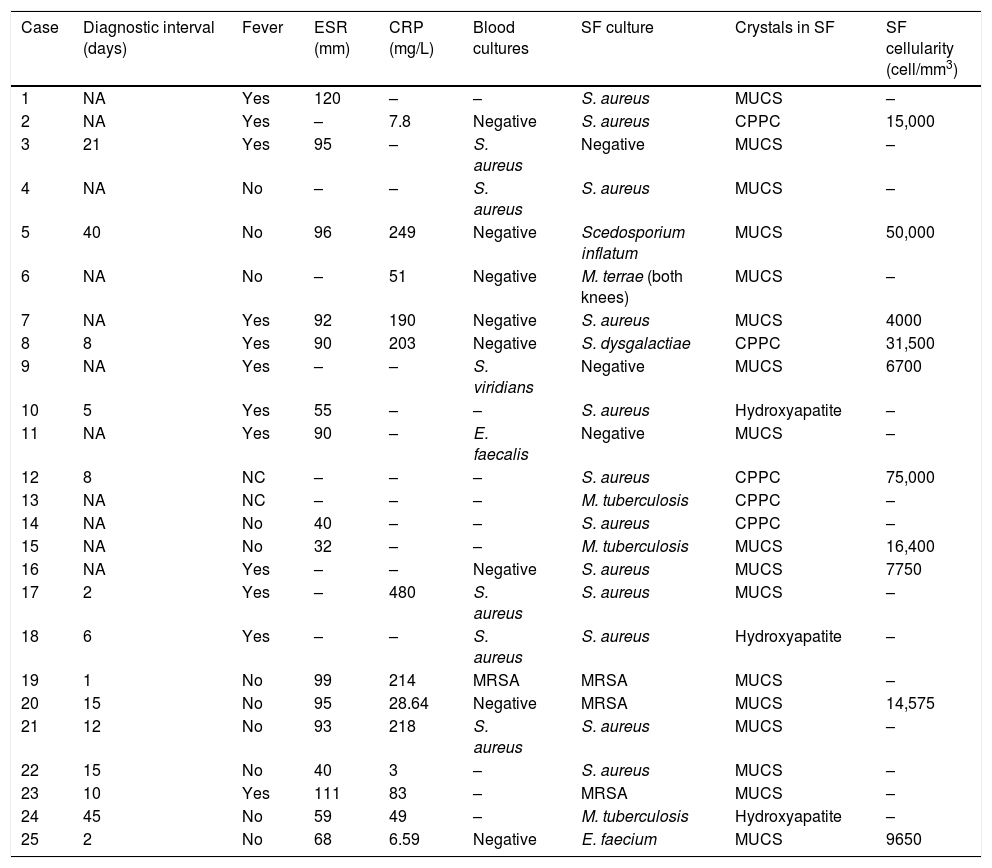
Monosodium urate crystals were most frequently identified in the cytological study of synovial fluid (17 cases; 68%), followed by calcium pyrophosphate dihydrate (CPPD) (5 cases; 20%), and hydroxyapatite (3 cases; 12%) (Table 2).
Clinical, microbiological and radiological characteristics of the series.
| Case | Diagnostic interval (days) | Fever | ESR (mm) | CRP (mg/L) | Blood cultures | SF culture | Crystals in SF | SF cellularity (cell/mm3) |
|---|---|---|---|---|---|---|---|---|
| 1 | NA | Yes | 120 | – | – | S. aureus | MUCS | – |
| 2 | NA | Yes | – | 7.8 | Negative | S. aureus | CPPC | 15,000 |
| 3 | 21 | Yes | 95 | – | S. aureus | Negative | MUCS | – |
| 4 | NA | No | – | – | S. aureus | S. aureus | MUCS | – |
| 5 | 40 | No | 96 | 249 | Negative | Scedosporium inflatum | MUCS | 50,000 |
| 6 | NA | No | – | 51 | Negative | M. terrae (both knees) | MUCS | – |
| 7 | NA | Yes | 92 | 190 | Negative | S. aureus | MUCS | 4000 |
| 8 | 8 | Yes | 90 | 203 | Negative | S. dysgalactiae | CPPC | 31,500 |
| 9 | NA | Yes | – | – | S. viridians | Negative | MUCS | 6700 |
| 10 | 5 | Yes | 55 | – | – | S. aureus | Hydroxyapatite | – |
| 11 | NA | Yes | 90 | – | E. faecalis | Negative | MUCS | – |
| 12 | 8 | NC | – | – | – | S. aureus | CPPC | 75,000 |
| 13 | NA | NC | – | – | – | M. tuberculosis | CPPC | – |
| 14 | NA | No | 40 | – | – | S. aureus | CPPC | – |
| 15 | NA | No | 32 | – | – | M. tuberculosis | MUCS | 16,400 |
| 16 | NA | Yes | – | – | Negative | S. aureus | MUCS | 7750 |
| 17 | 2 | Yes | – | 480 | S. aureus | S. aureus | MUCS | – |
| 18 | 6 | Yes | – | – | S. aureus | S. aureus | Hydroxyapatite | – |
| 19 | 1 | No | 99 | 214 | MRSA | MRSA | MUCS | – |
| 20 | 15 | No | 95 | 28.64 | Negative | MRSA | MUCS | 14,575 |
| 21 | 12 | No | 93 | 218 | S. aureus | S. aureus | MUCS | – |
| 22 | 15 | No | 40 | 3 | – | S. aureus | MUCS | – |
| 23 | 10 | Yes | 111 | 83 | – | MRSA | MUCS | – |
| 24 | 45 | No | 59 | 49 | – | M. tuberculosis | Hydroxyapatite | – |
| 25 | 2 | No | 68 | 6.59 | Negative | E. faecium | MUCS | 9650 |
CPPC: calcium pyrophosphate dihydrate crystals; MUCS: monosodium urate crystals; SF: synovial fluid; NA: not available; CRP: C-reactive protein; MRSA: methycillin-resistant Staphylococcus aureus; ESR: erythrocyte sedimentation rate.
The germs isolated in the synovial fluid were: methycillin-sensitive Staphylococcus aureus (12 cases; 48%), methycillin-resistant S. aureus (3 cases; 12%), Mycobacterium tuberculosis (3 cases; 12%), Scedosporium inflatum, Mycobacterium terrae, Streptococcus dysgalactiae, and Enterococcus faecium (one case each). Culture of the synovial fluid enabled the identification of the microorganism in all cases except 3 (12%), in which it was negative but the blood cultures were positive (S. aureus, Streptococcus viridans and Enterococcus faecalis in one case each). In 5 cases (20%), the blood cultures were positive for the same agent as that isolated in the synovial fluid. The mean±standard deviation (interval) of synovial fluid cellularity (data available for 10 cases) was 23,057.5±22,903.74 (4000–75,000)cells/mm3. Twelve patients (48%) had fever at the onset of clinical symptoms, and 2 (8%) septic shock in the first 72h from their arrival to hospital. The acute phase reagents at the time of diagnosis (C-reactive protein and erythrocyte sedimentation rate) were elevated in most cases: the mean±standard deviation (interval) of erythrocyte sedimentation rate was 79.69±26.90 (32–120)mm (data available in 16 cases), and the mean±standard deviation (interval) of C-reactive protein was 137.16±138.83 (3–480)mg/L (data available in 13 cases).
It was possible to recover information referring to the initial radiographs for 18 (72%) cases, which was normal in 6 (24%), with erosions in 7 (28%), joint impingement in 2 (8%), one case of isolated osteopenia, one case of osteitis, and one case of joint destruction (4%).
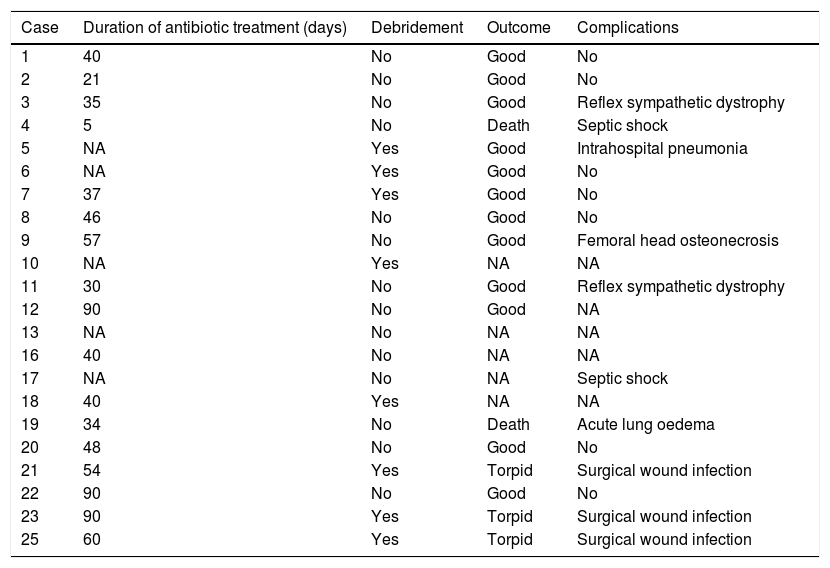
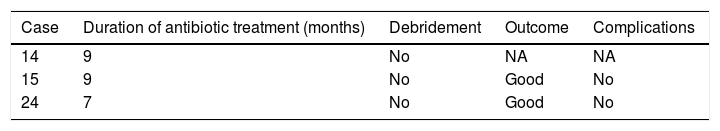
The mean duration±standard deviation (interval) of antibiotic treatment, excluding the cases caused by M. tuberculosis, was 48±24 days (5–90) (Table 3). The mean duration±standard deviation (interval) of antibiotic treatment for cases caused by M. tuberculosis was 8.3±1 months (7–9) (Table 4).
Treatment and outcome of patients (excluding those caused by Mycobacterium tuberculosis).
| Case | Duration of antibiotic treatment (days) | Debridement | Outcome | Complications |
|---|---|---|---|---|
| 1 | 40 | No | Good | No |
| 2 | 21 | No | Good | No |
| 3 | 35 | No | Good | Reflex sympathetic dystrophy |
| 4 | 5 | No | Death | Septic shock |
| 5 | NA | Yes | Good | Intrahospital pneumonia |
| 6 | NA | Yes | Good | No |
| 7 | 37 | Yes | Good | No |
| 8 | 46 | No | Good | No |
| 9 | 57 | No | Good | Femoral head osteonecrosis |
| 10 | NA | Yes | NA | NA |
| 11 | 30 | No | Good | Reflex sympathetic dystrophy |
| 12 | 90 | No | Good | NA |
| 13 | NA | No | NA | NA |
| 16 | 40 | No | NA | NA |
| 17 | NA | No | NA | Septic shock |
| 18 | 40 | Yes | NA | NA |
| 19 | 34 | No | Death | Acute lung oedema |
| 20 | 48 | No | Good | No |
| 21 | 54 | Yes | Torpid | Surgical wound infection |
| 22 | 90 | No | Good | No |
| 23 | 90 | Yes | Torpid | Surgical wound infection |
| 25 | 60 | Yes | Torpid | Surgical wound infection |
NA: not available.
The therapeutic combination that was used most in the days prior to definitive identification of the microorganism was cloxacillin (2g i.v. every 4h) combined with gentamicin (5mg/kg/day in one i.v. dose) over the first 48h.
The outcome of the joint infection was satisfactory in 14 (56%) cases, torpid in 3 (12%), and there were 2 deaths (8%): one (case 4) attributable to the infection (septic shock) in a patient with no previous risk factors, and another (case 19) due to acute lung oedema in a patient with previous mitroaortic valvulopathy. Six patients (24%) were lost to follow-up, and we do not have the data on their clinical outcome.
An arthrotomy was performed in 8 (36%) cases (excluding those caused by M. tuberculosis); in terms of complications, 3 patients (12%) had surgical wound infections, 2 (8%) reflex sympathetic dystrophy, 2 (8%) septic shock, intrahospital pneumonia, one had femoral head osteonecrosis, and one acute lung oedema. Nine (36%) patients had no complications.
DiscussionDifferential diagnosis of septic or crystal-induced arthritis is generally simple; however, it can become complicated when both entities present at the same time, which rarely occurs. Both patients with septic arthritis and those who develop crystal-induced arthritis can manifest fever, pain, rubor and joint swelling, therefore it is essential to perform an arthrocentesis with microbiological study of synovial fluid to establish a precise diagnosis. Regardless of whether or not the leucocyte count in synovial fluid is very high, or whether or not Gram staining shows microorganisms, it is imperative to take cultures (including mycobacterial cultures in cases of a more subacute course). This is more relevant for immunodepressed patients.9
There is little literature on the co-existence of septic arthritis with crystal deposition, and most are case reports. There are only 2 published case series, on a Taiwanese population.8,9 In the literature and this series there is a predominance of males (87%–93% in the series described).8,9 In our study, the percentage of males was somewhat lower (68%). The mean age of the patients varies (63.7 and 52.8 in the 2 series described).8,9 However, in our population it was rather higher (67 years). This could be explained by the difference in life expectancy between the Taiwanese population and the Spanish population, which is a little higher.
The most frequently described factors in the literature are the deposition of tophi, chronic kidney failure, diabetes mellitus, and liver cirrhosis.8,9 In our series, in addition to these factors, we found 4 cases with a previous kidney transplantation, and 2 cases who had undergone arthrocentesis or previous infiltration. However, we must stress that almost half the patients (40%) had no known risk factors.
As described in the literature, a third of the patients were afebrile at the time of diagnosis.8,9 In our series, 48% presented with fever. In the literature, only 36%–50% of the patients had positive blood cultures,8,9 therefore a high index of suspicion is required for correct diagnosis.
Both septic arthritis and crystal-induced arthritis can affect any joint. The most frequently affected joints according to the literature are the knee and the ankle.1–9 However, in this series, after the knee, the joints of the feet (first metatarsophalangeal and tarsus) were the second most frequently involved. With regard to the incidence of multiple joint involvement, the data from the series described in the literature vary greatly, with oligoarthritis between 10% and 78%, and polyarthritis between 0% and 7%.8,9 In our series, there were 2 cases (8%) with oligoarticular involvement, and no polyarticular involvement.
As in septic arthritis in the general population, methycillin-sensitive S. aureus is most commonly involved in the co-existence of crystal-induced and infectious arthritis, between 53% and 79% in the series described.8,9 In our case, S. aureus was responsible for 48% of the cases. However, methycillin-resistant S. aureus is becoming increasingly significant, and responsible for 12% of the cases in our series, and from 7.1%8 to 23.3%9 in the literature. We would highlight the percentage of cases caused by M. tuberculosis in our series (12%) compared to the cases described in the English-speaking literature, with only 3 publications on infectious arthritis caused by M. tuberculosis and concurrent arthritis caused by monosodium urate crystals,11–13 and no case of arthritis caused by CPPD. In our series we found 3 patients with infection by that microorganism, one with intercurrent gout, another with CPPD arthritis, and another with hydroxyapatite crystals in the synovial fluid study.
Most cases described in the literature (including the 2 case series8,9) describe the coexistence of gouty arthritis and infection.1,3–5,11–24 There are fewer cases described with CPPD.1,2,6,7,24–30 In our series, most patients also had monosodium urate crystals in the cytological study (68%), and there were fewer with CPPD (20%), and hydroxyapatite crystals (12%).
The outcome of joint infection is generally satisfactory if timely antibiotic treatment is started. Two of our patients died (8%). In the series described, the mortality was 28.6% and 6.6%.8,9 In both series surgical debridement was performed for almost half the patients (43% and 46.6%),8,9 while in our series this was performed in 36% of the cases. Noteworthy among the complications described in the Taiwanese series were a bilateral ankle amputation,8 and leg amputation due to necrosing fascitis.9 There were no serious local complications in our series.
To conclude, we must highlight that the coexistence of septic and crystal-induced arthritis is not commonly found in our environment, therefore it poses a diagnostic challenge and requires a high index of suspicion so that appropriate treatment can be started promptly, and a satisfactory outcome achieved. Microbiological study of synovial fluid is imperative in all cases of acute or subacute arthritis.
Conflict of interestsThe authors have no conflict of interest to declare.
Please cite this article as: Prior-Español Á, García-Mira Y, Mínguez S, Martínez-Morillo M, Gifre L, Mateo L. Coexistencia de artritis séptica y microcristalina: un reto diagnóstico. A propósito de 25 casos. Reumatol Clin. 2019;15:e81–e85.