There is strong evidence of a rise in cardiovascular risk in patients suffering from autoimmune diseases, especially in those with Systemic Lupus Erythematosus. Until now, there are a few trials that assess the potencial benefit of statins on the incidence of cardiovascular events and on lipid profile of patients with SLE. This evidence has not been synthesised and assessed altogether.
MethodsWe performed a search in databases of literature published until August of 2016 (Embase, MEDLINE, Cochrane Library, SciELO, Clinical Evidence, DynaMed, Cochrane Central Register of Controlled Trials, LILACS), identifying controlled clinical trials that could estimate the impact of statins on mortality, cardiovascular events, C-reactive protein and lipid profile in patients with Systemic Lupus Erythematosus. The quality of the information available was assessed with a meta-analysis, using a random effects model, employing the RevMan 5.3 software.
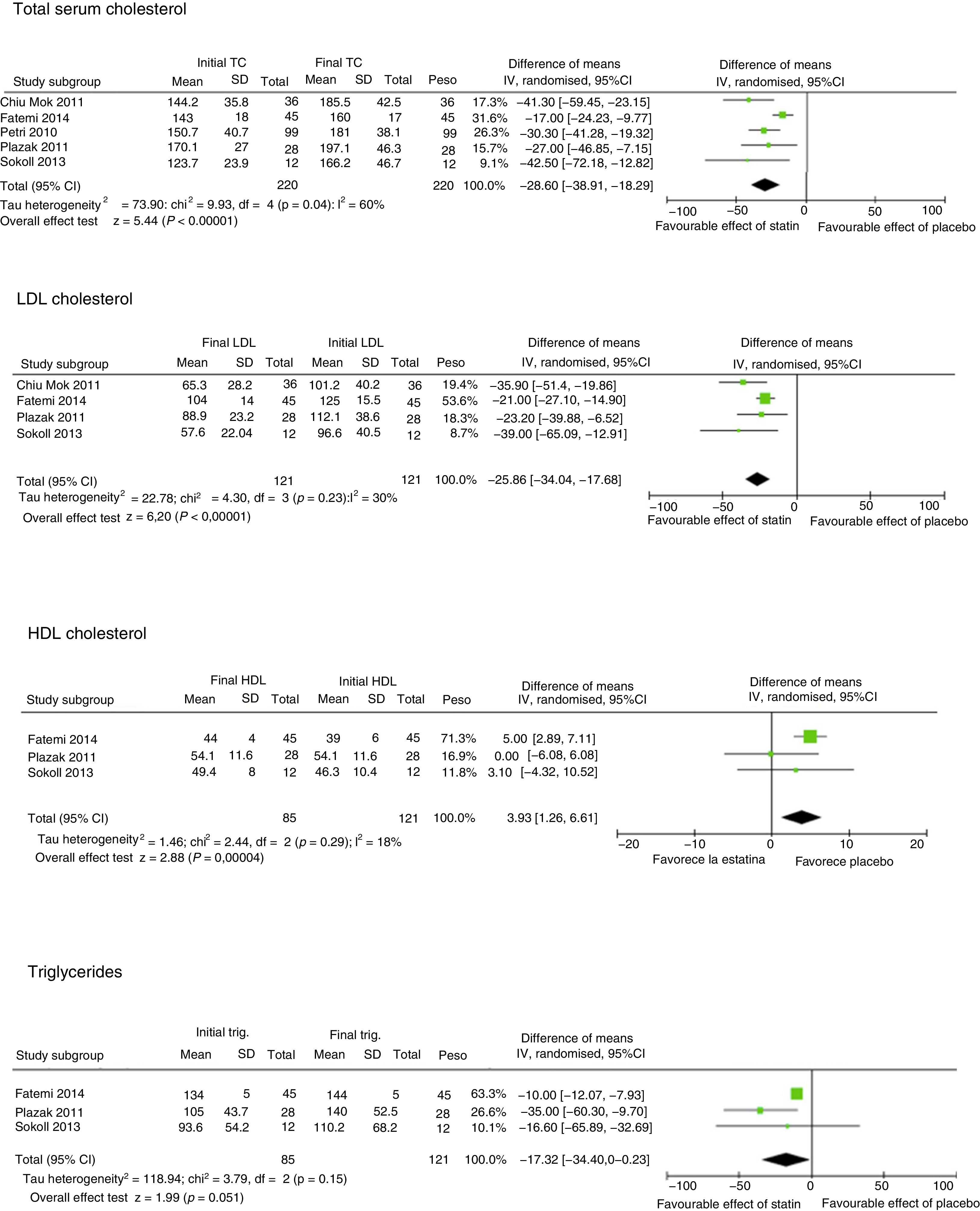
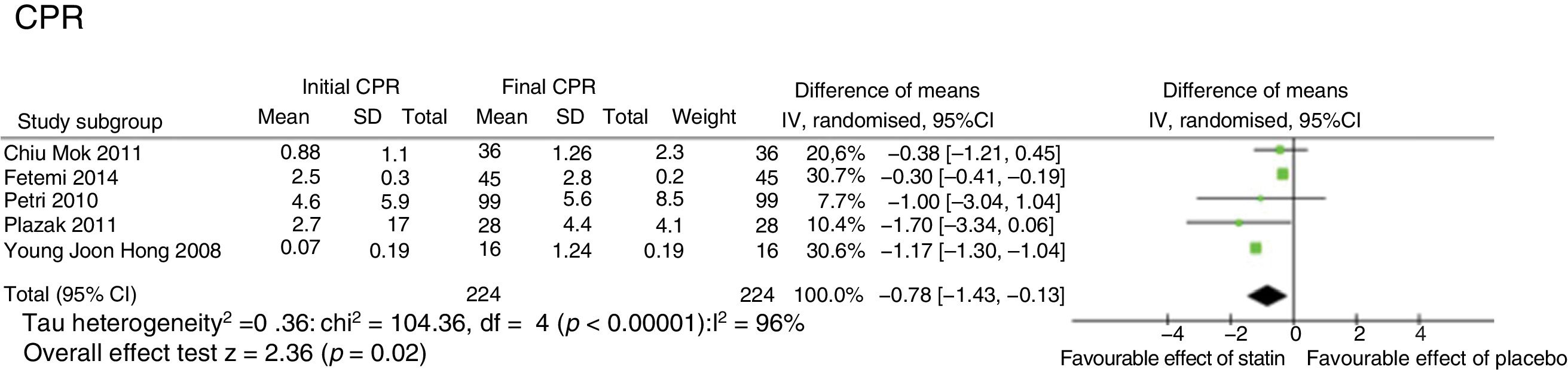
Results6 trials and 412 patients were included in the analysis. The use of statins in patients with SLE was found to significantly reduce the levels of serum total cholesterol (mean difference [MD] −31.4mg/dL; CI 95% −43.0; −19.9), and serum low density cholesterol (MD −31.4mg/dL; IC 95% −43.0; −19.9), but had no impact on levels of serum triglycerides (MD 4mg/dL; IC 95% 2.49; 6.21) and C-reactive protein (MD −0.78; IC 95% −1.43; −0.13). No evidence was found about the impact on the risk of mortality or cardiovascular events.
ConclusionStatins have a significant effect on the levels of serum total cholesterol, LDL cholesterol and C-reactive protein, however, more randomised controlled trials with long-term follow-up are necessary to assess the impact on mortality and cardiovascular risk.
Existe evidencia que muestra un aumento del riesgo cardiovascular en pacientes que padecen de enfermedades autoinmunes, en particular, de lupus eritematoso sistémico. Hasta el momento existen pocos estudios que evalúen el potencial beneficio de las estatinas en la incidencia de eventos cardiovasculares y en el perfil lipídico de pacientes con, y esta evidencia no ha sido sintetizada y evaluada en conjunto.
MétodosSe realizó una búsqueda de la literatura hasta agosto de 2016 (Embase, MEDLINE, Cochrane Library, SciELO, Clinical Evidence, DynaMed, registro de experimentos clínicos de Cochrane, LILACS), identificando ensayos clínicos controlados que evaluaran el impacto de las estatinas en mortalidad, eventos cardiovasculares, proteína C reactiva y perfil lipídico en pacientes con lupus eritematoso sistémico. Se evaluó la calidad de la información disponible y se metaanalizó utilizando un modelo de efectos aleatorios, utilizando el programa RevMan 5.3.
ResultadosUn total de 6 estudios y 412 pacientes fueron incluidos para el análisis. Se encontró que el uso de las estatinas en paciente con LES reduce significativamente los niveles de colesterol total (diferencia de medias [DM] −31,2mg/dL; IC 95% −41,9; −20,5), colesterol de baja densidad (DM −31,4mg/dL; IC 95% −43,0; −19,9), sin impacto en los niveles de triglicéridos (DM 4mg/dL; IC 95% 2,49; 6,21) y proteína C reactiva (DM −0,78; IC 95% −1,43; −0,13). No se encontró ninguna evidencia sobre impacto en el riesgo de mortalidad o eventos cardiovasculares.
ConclusiónLas estatinas tienen un impacto significativo en los niveles de colesterol total, colesterol unido a lipoproteínas de baja densidad y proteína C reactiva, sin embargo, son necesarios nuevos estudios aleatorizados controlados con seguimiento a largo plazo para evaluar el impacto en la mortalidad y el riesgo cardiovascular.
There is evidence of increased morbidity and mortality in patients with systemic lupus erythematosus (SLE) of cardiovascular cause. In the last 3 decades overall mortality has decreased; however, there has been an increase in cause-specific cardiovascular mortality.1
SLE is the prototype of autoimmune disease. In recent years cardiovascular disease has replaced infections as the first cause of mortality in these patients, principally due to the more rational use of corticosteroids and immunosuppressive drugs, which has resulted in longer life expectancy.1 it would seem that the pathophysiological basis for this high cardiovascular mortality is the premature development of accelerated atherosclerosis.2–4 In the Systemic Lupus Erythematosus Disease Activity Index – SLEDAI – score that reflects the activity of the disease over a year, higher scores correlate with a 5% increase in cerebrovascular risk at 2 years, and an increased risk of lupus nephritis that is associated with an increased risk of cardiovascular events of up to 50%.5
Statins are considered the essential pharmacological strategy demonstrated to reduce the risk of cardiovascular events. Statins enable regulation of the inflammatory process associated with the development of atherosclerosis. They also have immunomodulatory properties, and have a direct impact on morbidity and mortality in primary and secondary prevention.6 However, as yet, the systematic use of statins in patients with SLE to modulate cardiovascular risk has not been proven to have significant impact on cardiovascular events in individual studies, and therefore remains a controversial strategy.6–8 By means of a systematic review we shall evaluate the impact of the use of statins for patients with SLE, and the modification of their cardiovascular risk.
MethodsA systematic review was performed to evaluate the impact of the use of statins on lipid profile, C-reactive protein (CRP) and on the reduction of cardiovascular events. Studies were selected that included adults over the age of 18, with a diagnosis of SLE, defined by meeting 6 of the 11 criteria of the American College of Rheumatology or 4 of the criteria of the Systemic Lupus International Collaborating Clinics of 2012.9
The intervention was defined as the use of statins (atorvastatin, rosuvastatin, pravastatin or simvastatin) for primary and secondary prevention. The use of a placebo or different statin was accepted as the control group. The outcomes of interest were: death due to any cause, death of cardiovascular cause, acute myocardial infarction, cerebrovascular accident, myocardial revascularisation, modified lipid fractions and CPR levels, and the presence of side or adverse effects.
The search was performed in each of the databases from the start up until August 2016 (Embase, MEDLINE, Cochrane Library, SciELO, Clinical Evidence, DynaMed, Cochrane Register registry of clinical trials, LILACS), limiting the studies to randomised clinical trials published in English and Spanish. The search strategy was to use the following MeSH terms: Lupus Erythematosus, Systemic, Hydroxymethylglutaryl-CoA Reductase Inhibitors, Pravastatin, Simvastatin, Rosuvastatin, atorvastatin.
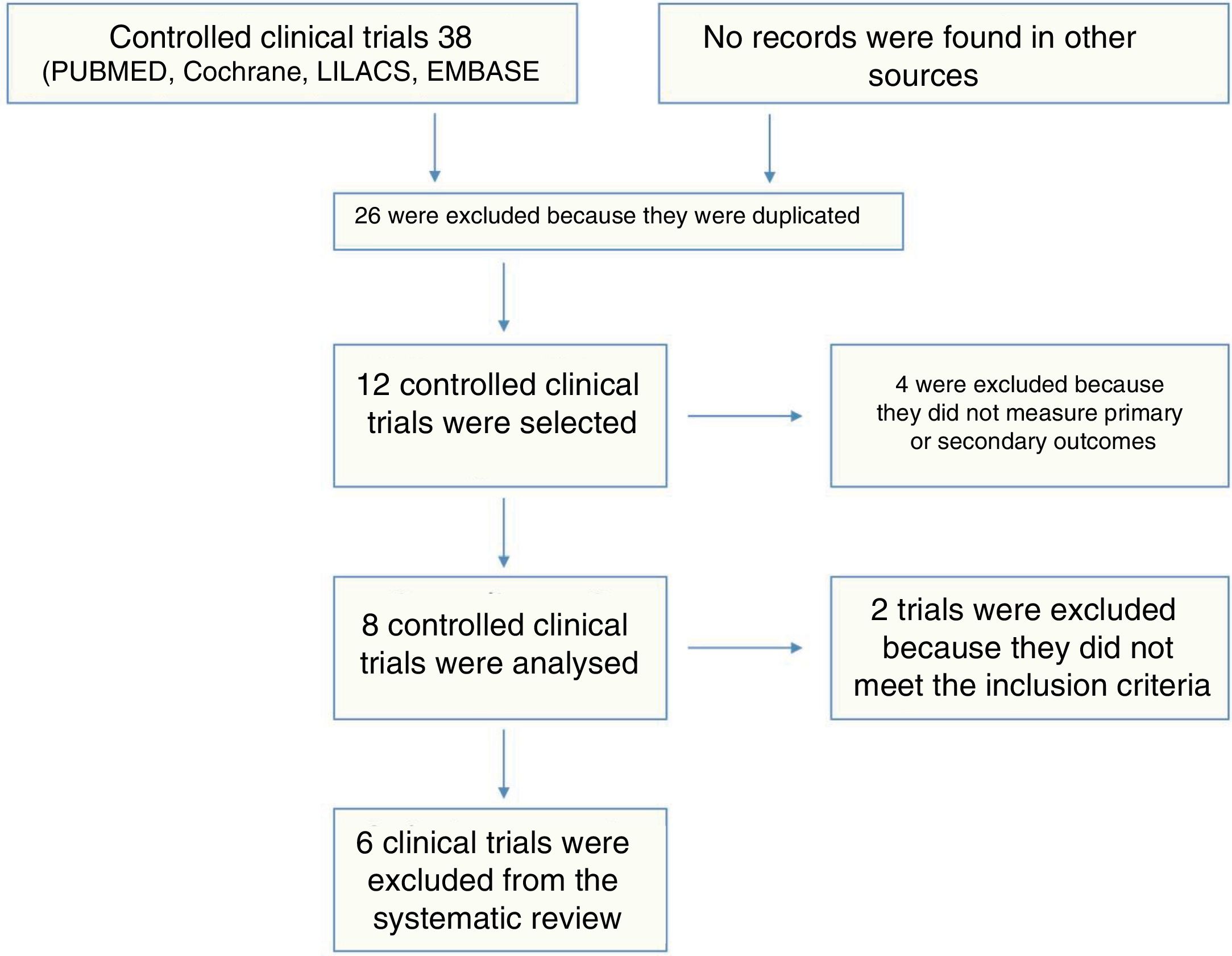
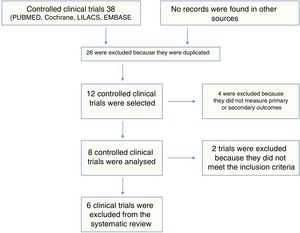
The studies were selected by 2 referees who studied titles and abstracts, any differences were resolved by a third assessor. Fig. 1 shows the search results. Similarly, the data were extracted independently by 2 investigators (PS and ET), using a standardised format. Any discrepancies were resolved by consensus, with the intervention of a third investigator (OMMV). The investigators collected the following data for each study: sample size, baseline characteristics of the population, criterion used to diagnose SLE, presence of comorbidities (diabetes mellitus, arterial hypertension, smoking), drug used and dose, duration of follow-up and results on outcomes, including the number of events, and summary estimate used.
The quality of the clinical trials was assessed according to the Scottish Intercollegiate Guidelines Network checklist.10 The probability of each potential bias was evaluated by 2 referees who worked independently. Any disagreements were resolved by discussion, and the participation of a third referee.
Review Manager 5.3 was used for summary and meta-analysis of the information, and estimate of heterogeneity (I2 calculation), as recommended by the Cochrane Collaboration. A model of randomised effects was used, which that enabled an evaluation of the heterogeneity between the different studies included. Similarly, the clinical analysis of the heterogeneity found is presented where considered clinically relevant.
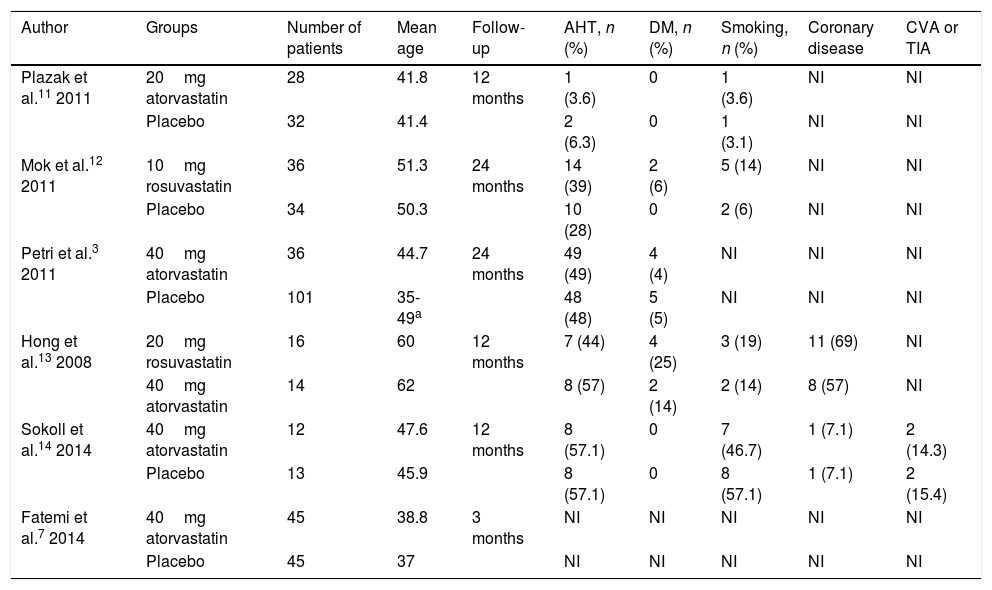
ResultsA total of 38 studies were identified, of which 32 were excluded (26 were duplicated, 4 included patients under the age of 18, and 2 reported none of the outcomes of interest). Six studies with a total of 412 patients included were considered for the final analysis.3,7,11–14 Of the 6 articles on randomised clinical trials; 5 used high potency statins (rosuvastatin or atorvastatin) compared to a placebo, and only one study compared 2 statins.13 The characteristics of the patients included in each of the studies, including the frequency of comorbidities, are shown in Table 1.
Studies included.
| Author | Groups | Number of patients | Mean age | Follow-up | AHT, n (%) | DM, n (%) | Smoking, n (%) | Coronary disease | CVA or TIA |
|---|---|---|---|---|---|---|---|---|---|
| Plazak et al.11 2011 | 20mg atorvastatin | 28 | 41.8 | 12 months | 1 (3.6) | 0 | 1 (3.6) | NI | NI |
| Placebo | 32 | 41.4 | 2 (6.3) | 0 | 1 (3.1) | NI | NI | ||
| Mok et al.12 2011 | 10mg rosuvastatin | 36 | 51.3 | 24 months | 14 (39) | 2 (6) | 5 (14) | NI | NI |
| Placebo | 34 | 50.3 | 10 (28) | 0 | 2 (6) | NI | NI | ||
| Petri et al.3 2011 | 40mg atorvastatin | 36 | 44.7 | 24 months | 49 (49) | 4 (4) | NI | NI | NI |
| Placebo | 101 | 35-49a | 48 (48) | 5 (5) | NI | NI | NI | ||
| Hong et al.13 2008 | 20mg rosuvastatin | 16 | 60 | 12 months | 7 (44) | 4 (25) | 3 (19) | 11 (69) | NI |
| 40mg atorvastatin | 14 | 62 | 8 (57) | 2 (14) | 2 (14) | 8 (57) | NI | ||
| Sokoll et al.14 2014 | 40mg atorvastatin | 12 | 47.6 | 12 months | 8 (57.1) | 0 | 7 (46.7) | 1 (7.1) | 2 (14.3) |
| Placebo | 13 | 45.9 | 8 (57.1) | 0 | 8 (57.1) | 1 (7.1) | 2 (15.4) | ||
| Fatemi et al.7 2014 | 40mg atorvastatin | 45 | 38.8 | 3 months | NI | NI | NI | NI | NI |
| Placebo | 45 | 37 | NI | NI | NI | NI | NI |
CVA: cerebrovascular accident; TIA: transient ischaemic attack; DM: diabetes mellitus; AHT: arterial hypertension; NI: no available information.
Patients with systemic lupus erythematosus per criteria.
No data on mortality, AMI, CVA or requirement for myocardial revascularisation were found in any of the studies included in the analysis. We should clarify that the maximum follow-up in these studies was 24 months.
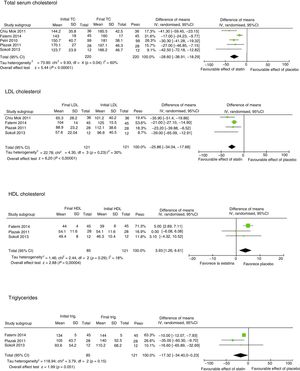
Lipid fractions and C-reactive protein levelFive studies evaluated the impact of statins compared to a placebo on the lipid profile3,7,11,12,14 (Fig. 2). All of them evaluated the impact on total serum cholesterol levels (TC). The mean TC reduction was 31.2mg/dL (95%CI −41.9; −20.5; I2 60%), this finding was consistent in all the studies. Four studies assessed the impact of statins on low-density lipoprotein cholesterol (LDL-C) levels.7,11,13,14 A significant reduction of 31.4mg/dL (95%CI −43; −19.9; I2 30%) was seen. Three studies assessed high-density cholesterol (HDL-C),7,11,14 and found another statistical increase that was not clinically significant of 4mg/dL (95%CI 2.49; 6.21; I2 18%),these finding had no significant heterogeneity.
Impact of statins on lipid fractions.
Total serum cholesterol, Initial TC, Final TC, Study subgroup, Mean, SD, Difference of means, IV, randomised, 95%CI, Tau heterogeneity, Overall effect test, Favourable effect of statin, Favourable effect of placebo, LDL cholesterol, Initial LDL, Final LDL, HDL cholesterol, Initial HDL, Final HDL, Triglycerides, Initial trig., Final trig., Weight.
Five studies evaluated the impact of statins on CPR levels3,7,11,12,14 (Fig. 3), showing a statistical decrease that was not clinically significant of .78mg/dL (95%CI −1.43; −.13; I2 98%).
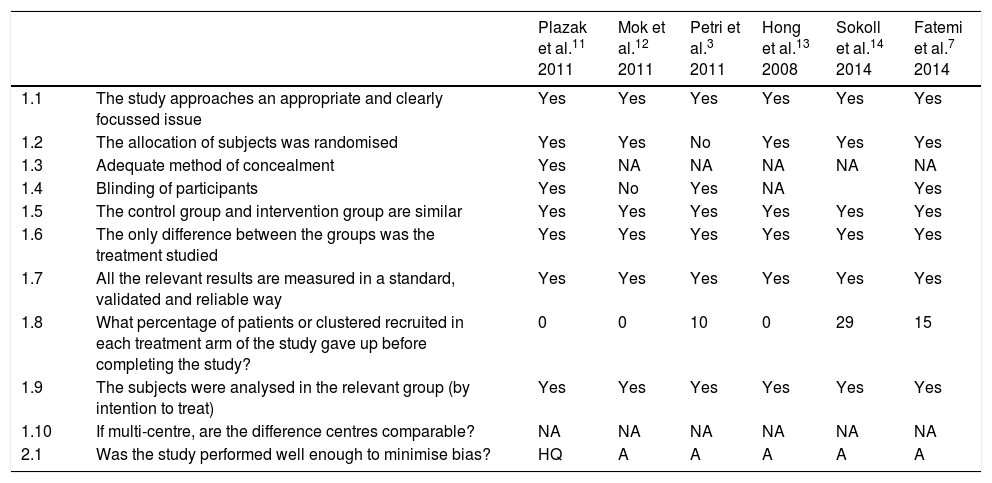
Quality of the studiesWhen checking the quality of the clinical studies analysed to assess the impact of statins on overall cardiovascular risk, we found that only one11 used appropriate blinding of randomisation. In 3 of the studies there was no blinding of the patients included.12–14 The study by Petri et al. of 20113 did not record the blinding procedure (Table 2).
Quality of the studies.
| Plazak et al.11 2011 | Mok et al.12 2011 | Petri et al.3 2011 | Hong et al.13 2008 | Sokoll et al.14 2014 | Fatemi et al.7 2014 | ||
|---|---|---|---|---|---|---|---|
| 1.1 | The study approaches an appropriate and clearly focussed issue | Yes | Yes | Yes | Yes | Yes | Yes |
| 1.2 | The allocation of subjects was randomised | Yes | Yes | No | Yes | Yes | Yes |
| 1.3 | Adequate method of concealment | Yes | NA | NA | NA | NA | NA |
| 1.4 | Blinding of participants | Yes | No | Yes | NA | Yes | |
| 1.5 | The control group and intervention group are similar | Yes | Yes | Yes | Yes | Yes | Yes |
| 1.6 | The only difference between the groups was the treatment studied | Yes | Yes | Yes | Yes | Yes | Yes |
| 1.7 | All the relevant results are measured in a standard, validated and reliable way | Yes | Yes | Yes | Yes | Yes | Yes |
| 1.8 | What percentage of patients or clustered recruited in each treatment arm of the study gave up before completing the study? | 0 | 0 | 10 | 0 | 29 | 15 |
| 1.9 | The subjects were analysed in the relevant group (by intention to treat) | Yes | Yes | Yes | Yes | Yes | Yes |
| 1.10 | If multi-centre, are the difference centres comparable? | NA | NA | NA | NA | NA | NA |
| 2.1 | Was the study performed well enough to minimise bias? | HQ | A | A | A | A | A |
A: acceptable; HQ: high quality; NA: not applicable.
It is evident that statins (atorvastatin and rosuvastatin) have a clinical and statistically significant benefit in the reduction of TC and LDL-C. This is a consistent finding with many studies that evaluate the efficacy of statins,15 with a modest increase in HDL-C, the clinical relevance of which is not known, although it is statistically significant. This systematic review found no information on cardiovascular events and mortality in this specific population, possibly due to the lack of studies directed at specifically assessing cardiovascular events in this population, and the possible reduction of the risk with the use of statins. This might be because this is a younger population with a shorter exposure time to acquired cardiovascular risk factors. Likewise, it is notable that better control of the disease has been achieved in recent years, with a reduction in the number of episodes and severity of relapse, which increases the survival of patients with SLE, and reduces exposure to the inflammatory processes associated with shorter exposure to immunosuppressive and anti-inflammatory therapies and a lower dose of steroids, which in turn reduces this population's potential increased cardiovascular risk.
Although there is valuable information on the favourable effect of statins (lipid-lowering effect) on lipid fraction levels, there is no evidence on pleiotropic potentials (anti-inflammatories and antioxidants) that might play an interesting pathophysiological role in the control of the disease, and the reduction of cardiovascular events.
The principal limitations of the study were the follow-up time which, although appropriate to demonstrate the benefit on the reduction of lipid fractions, is short to assess the impact on the presentation of cardiovascular events. Although the population at high cardiovascular risk is known, and “the lower the better” has been verified with regard to LDL-C levels, the information we found does not enable us to confirm this relationship, but extrapolating this information to patients at high risk of cardiovascular disease could, in theory, be feasible, and uncertain for people at low cardiovascular risk or with a cardiovascular risk within the population average. Similarly, none of the studies reported variations in all lipid fractions (incomplete data for HDL-C and TG).
We consider the systematic review to be a valuable contribution in terms of the wide gap in research on a topic as important as cardiovascular risk in patients with SLE. If this situation is tackled great progress will be made in reducing morbidity and mortality in the first years following a diagnosis of SLE.
There is absolutely no doubt that patients with SLE are at real cardiovascular risk. Its impact on morbidity and mortality associated with cardiovascular events is so great that it is now considered the main cause of death after the renal complications in the initial stages of the disease. Although insufficient information is available to support the routine use of statins for patients with SLE, we have come a long way in the search for responses that can minimise the impact of cardiovascular risk in these patients.
ConclusionsThe use of statins for patients with SLE has a favourable and statistically significant effect on the reduction of TC and LDL-C, and shows a slight increase in HDL-C levels; no statistically significant effects were found on the reduction of CRP. There is insufficient information to analyse the effect of the use of statins in reducing cardiovascular events and mortality.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Sánchez P, Toro-Trujillo E, Muñoz-Velandia OM, García AA, Fernández-Ávila DG. Impacto terapéutico de las estatinas en el perfil lipídico y el riesgo cardiovascular en pacientes con lupus eritematoso sistémico: revisión sistemática de la literatura y metaanálisis. Reumatol Clin. 2019;15:e86–e91.