To describe the results of the comparative study between both versions of an immunoassay commercialized for therapeutic drug monitoring of adalimumab (ADA) in rheumatoid arthritis (AR).
Material and methods140 samples of patients with RA treated with ADA 40mg every 14 days were analyzed by both versions of the test (V1 or previous and V2 or updated).
ResultsA good correlation was obtained by both versions. In general V2 provides higher results of ADA's concentration than V1 and presents greater precision in the range of concentrations for clinical decisions, adjusting for the real concentration of the drug in blood. In addition, V2 allows for complete automation, which simplifies the analysis and reduces significantly the variability.
ConclusionADA's monitoring with the updated version demonstrated to have technical significant advantages, constituting a more practical tool for therapeutic decisions in patients with RA.
Describir los resultados del estudio comparativo entre las 2 versiones de un inmunoanálisis comercializado para la monitorización terapéutica de adalimumab (ADA) en artritis reumatoide (AR).
Material y métodosSe han analizado 140 muestras de suero de pacientes con AR tratados con ADA 40mg cada 14 días con las 2 versiones del ensayo (V1 o anterior y V2 o actualizada).
ResultadosSe obtuvo una buena correlación con las dos versiones. En general, V2 proporciona resultados más altos de concentración de ADA que V1 y presenta una mayor precisión en el rango de concentraciones próximas al nivel de decisión clínica, ajustándose más a la concentración real del fármaco en sangre. Además, permite la automatización completa, lo cual simplifica mucho el análisis, y reduce significativamente la variabilidad.
ConclusiónLa monitorización de ADA con la versión actualizada demostró tener ventajas técnicas significativas, pudiendo ser una herramienta más práctica para la toma de decisiones terapéuticas en pacientes con AR.
Adalimumab (ADA,® Humira, Abbott Laboratories, North Chicago, Illinois, USA) is a fully human monoclonal antibody that specifically binds to tumor necrosis factor α (TNΦ–α) neutralizing its biological function and modulating its response.Despite its proven efficacy widely adopted in different clinical indications, some patients do not respond or have a loss of response over time. One possible explanation is that, at steady state, serum ADA levels do not necessarily ensure that effectiveness is achieved. In some cases this has been associated with the presence of anti-ADA antibodies that form complexes with,1,2 ADA, increasing its clearance. Furthermore, quantification of therapeutic levels of ADA at the end of the dosing interval in non-responders provides valuable information in the subsequent selection of the new treatment.3 Also, the development of dose-response curves can lead to dose spacing of this drug in patients in clinical remission.4
Until now, decisions in these cases were based solely on the clinical course of the patient. However, there is consistent and gradually increasing literature showing that the drug level measurements and anti-drug antibodies are clinically relevant for the individualization of treatment.5
As of 2 years ago an enzyme immunoassay (ELISA) for the quantification of serum-free ADA and anti-ADA antibodies (Promonitor® Proteomika SL, distributed by Menarini Diagnostics SA®) is marketed in our country with precision, linearity and clinical validation criteria suitable for therapeutic drug monitoring of ADA.6,7 Recently, the manufacturer has released a new version with significant changes regarding the practicability of the analytical assay.
The objective of this paper is to describe the results of the comparative study between the 2 versions of ELISA marketed for therapeutic drug monitoring of ADA in patients with rheumatoid arthritis (RA).
Materials and MethodsWe have selected 140 serum samples from patients with RA treated with ADA40mg every 14 days, with different drug concentrations and anti-drug antibodies, so that the entire analytical range of the new technique is covered (from 0.024 to 12mg/L and 3.5–2000AU/ml). For each patient a sample of 5mLserum was obtained before subcutaneous drug administration and stored frozen at -80° C until analysis in duplicate with 2 versions of ELISA, following the conditions specified by the manufacturer.
In the first version of the assay (V1), for the determination of levels of ADA, a plate was coated with TNΦ–α immobilized by a monoclonal antibody in a first incubation. And for the determination of anti-ADA antibodies, the samples were added to the wells with prior drug immobilization. After incubation with the patient sample, in both cases, the detection was carried out using a biotin-labeled monoclonal antibody and the concentration was determined by colorimetric reaction (450nm). Calibration curves were constructed with 10-fold dilutions of the standards (0.156–40ng/mL for ADA and 0.4–100AU/ml for anti-ADA antibodies), and each sample underwent 6 serial dilutions (1/10–1/10.240), in order to assure readings within the linear part of the calibration curve.
In the updated version (V2), the calibration range is larger: 1.25–60ng/mL and 3.13–200AU/mL for the quantification of ADA and anti-ADA antibodies, respectively. Dilutions per patient were reduced to 2 (1/10 and 1/200 for ADA, and undiluted and 1/10 for anti-ADA antibodies) and the labeled enzyme becomes peroxidase conjugated with streptavidin.
Interassay precision was calculated using the coefficient of variation. The Student's t test was employed for paired samples and was conducted to compare the concentrations of ADA between the 2 analyses with the same version of the test and using a Kappa statistic of agreement assessed following the categorization of results. With the correlation analysis, the relationship between the measurements with the two versions of the test was performed. The concordance correlation coefficient (CCC),8 and confidence intervals were calculated, assessing the average difference over the entire range of magnitudes measured by the Bland–Altman plot.9
ResultsThe reproducibility of the new version of the assay was determined by processing 20 samples in 3 different nonconsecutive days using 2 different lots of reagent. Interassay imprecision was, on average, of 12.5%, showing an acceptable reproducibility.
Discrepancies between repetitions were evaluated by analyzing 30 samples of ADA in duplicate with each of the versions of the assay. Significant differences between the two measurements with V1 (P<.001) were observed, but no significant difference with V2 (P=.139) was observed. When categorizing measurement ranges (0–3, 3–7, 7–12 and more than 12mg/L), a low correlation between the concentrations obtained with ADA V1 (Kappa 0.14 [0–0 observed, 59]) and moderate to high concordance with ADA V2 (Kappa 0.72 [0.44–0.86]).
To assess which of the two versions gives the most adjusted values to the actual concentration of the drug, we compared ADA quantification in 26 samples of known doped serum drug concentrations between 0.005 and 2.0mg/L. The average percentage of recovery relative to the theoretical concentration was 42 and 85% for V1 and V2, respectively, showing that V2 is more accurate and more closely reflects the amount of ADA in the sample.
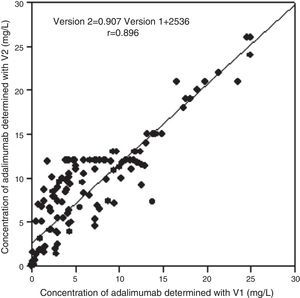
The comparison study between the 2 versions of the ADA assay (n=140) gives a correlation coefficient of 0.896 and a CCC of 0.85 (confidence interval 95%, 0.80–0.89) (Fig. 1). The Bland–Altman analysis shows a good agreement between the two tests (bias=2.0 [2DE: −7.8 to 3.8]) (Fig. 2).
Comparison study by correlation analysis of adalimumab concentrations determined with the 2 versions of marketed kits (n=140). A correlation coefficient of 0.896 (Version 2=0.907, Version 1 2536+) and a concordance correlation coefficient of 0.85 (95% CI: 0.80–0.89) has been obtained. gr1 Version Version Y: Concentration of adalimumab determined with V2 X: Concentration of adalimumab determined with V1.
Bland–Altman analysis for adalimumab concentrations determined with the 2 versions of the test marketed. The average difference over the entire range of measured magnitudes is evaluated, resulting in a good agreement between the two tests (bias=2.0 [2DE: −7.8 to 3.8]). gr2 Y: Concentration of adalimumab X: Mean concentration of adalimumab determined with V1 and V2.
In the ELISA for detection of anti-ADA we obtained a quantitative linear correlation (r=0.994) between measurements with two versions of the test. The agreement was 100% for the 16 samples tested with V1 that were positive to anti-ADA antibodies and also detected in other 4 samples with V2, showing greater sensitivity with the updated version.
DiscussionThe fundamental objective of the monitoring of drug therapy is to improve patient care and therapy through dose adjustment based on drug plasma concentrations.10 Therapeutic monitoring of ADA is seen as an essential tool to ensure efficient use of this drug, since clearance differs significantly between individuals and, in time, other factors that can alter its elimination are unknown.11,12 Combined with other clinical data, it provides useful information allowing adjustment of dose in each patient in a guided manner, ensuring optimal therapeutic effect and limiting toxicity.13,14
Several research groups have developed different assay formats for ADA monitoring with their own advantages and disadvantages (ELISA, RIA, cellular assays). But for now, there is no comprehensive comparative study of the various tests which in some cases have shown discrepancies between platforms, highlighting the need for standardization.15
Of all the techniques available, ELISA is the most widely used for its ease of application in clinical practice. The first version of the test evaluated in this study consisted of multiple manual steps and each of them could be the source of analytical variability: from the covering of the wells of the plate to the preparation of calibrators, reagents and samples. In the new version parameters that can induce variability in the results are limited to the maximum: the wells are precovered presented, calibrators and reagents prediluted and sample dilutions restricted for optimal reading.
In the comparative trial a good correlation between measurements of ADA and anti-ADA antibodies with two versions of the test are obtained. In general, V2 provides higher ADA concentration results than V1 and it has a higher accuracy in the range of concentrations near the clinical decision level, being better adjusted to the actual concentration of the drug in blood. In addition, in the new version of the ELISA assay, time is significantly reduced from 6 to 2.5h which allows full automation, greatly simplifying the analysis and significantly reducing the variability in repetitions of the samples something recommended for routine use in the clinical laboratory. Still, we must remember that the test results may be influenced by other difficult to control factors and which may affect the development of any ELISA.
Assuming the inherent limitations of this technique, with the availability of this new commercial version for therapeutic monitoring of ADA, the provision of reliable data for making therapeutic decisions in patients with RA is facilitated. It is necessary to work on the standardization and validation of assays, to reach consensus on the interpretation of drug concentrations and anti-drug antibodies, establishing the therapeutic window for each indication, and treatment algorithms to design evidence-based data validated in clinical practice.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that no experiments have been performed on humans or animals.
Data confidentialityThe authors declare that they have followed the protocols of their workplace regarding the publication of data from patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained informed consent from patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
FinancingThe study was supported by a research grant by the Association for Research in Rheumatology of Marina Baixa (AIR-MB).
Conflict of InterestThe authors declare no conflict of interest in connection with this work.
Grupo AIRE-MB-HGM: Asociación para la Investigación en Reumatología de la Marina Baixa (AIRE-MB): José Rosas, Esteban Salas, José Miguel Senabre-Gallego, Gregorio Santos-Soler (S. Reumatología, Hospital Marina Baixa), Francisca Llinares-Tello, Juan Molina (S. Laboratorio, Hospital Marina Baixa); Carlos Santos-Ramírez (S. Reumatología, Hospital Marina Alta, Denia), Xavier Barber (CIO-Universidad Miguel Hernández, Elche), Mabel Sánchez-Barrioluengo (INGENIO [SIC-UPV], Universitat Politècnica de València).
Hospital Universitario Gregorio Marañón (HUGM): Inmaculada de la Torre, Lara Valor, Diana Hernández, Luis Carreño (S. Reumatología).
The names of the components of the AIR-MB-HUGM Group are listed in Annex 1.
Please cite this article as: Llinares-Tello F, Rosas J, de la Torre I, Valor L, Barber X, Senabre JM, et al. Estudio comparativo de las 2 versiones de un inmunoanálisis comercializado para la monitorización terapéutica de adalimumab en artritis reumatoide. Reumatol Clin. 2014;10:105–108.





![Bland–Altman analysis for adalimumab concentrations determined with the 2 versions of the test marketed. The average difference over the entire range of measured magnitudes is evaluated, resulting in a good agreement between the two tests (bias=2.0 [2DE: −7.8 to 3.8]). gr2 Y: Concentration of adalimumab X: Mean concentration of adalimumab determined with V1 and V2. Bland–Altman analysis for adalimumab concentrations determined with the 2 versions of the test marketed. The average difference over the entire range of measured magnitudes is evaluated, resulting in a good agreement between the two tests (bias=2.0 [2DE: −7.8 to 3.8]). gr2 Y: Concentration of adalimumab X: Mean concentration of adalimumab determined with V1 and V2.](https://static.elsevier.es/multimedia/21735743/0000001000000002/v2_201403290120/S2173574313001378/v2_201403290120/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w937trqSwLGgTrQM2QjUSRyU=)



