To assess whether there are any real-life differences between ankylosing spondylitis (AS) patients treated with NSAID or TNF inhibitors (TNFi) regarding disease activity.
MethodsThis is an observational transversal unicentric study with retrospective retrieval of data from clinical records of all AS patients attended in our hospital. We compared clinical activity measured by Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) scores between patients treated with NSAID and those treated with TNFi, in terms of low disease activity defined as BASDAI<4, and inactivity when BASDAI≤2. As secondary variables, we also collected epidemiological, clinical and radiological data from all those patients.
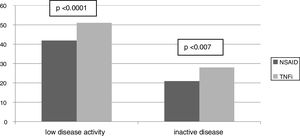
ResultsA total of 152 AS patients (81% male), with an average age of 49.45±12.38 years and a disease duration of 13.5±9.79 years were included in the study. Eighty-nine patients (58.6%) were treated with NSAID and 63 (41.4%) with TNFi. The proportion of patients with low disease activity and inactive disease was significantly higher in the TNFi treatment group compared to the NSAID group (81 vs. 47, P=.0001) and (44 vs. 24, P=.007), respectively. Patients treated with NSAIDs also showed significantly more global pain and night pain than those under TNFi therapy. The BASFI score and especially the type of treatment (NSAID or TNFI) were the only variables independently associated with low disease activity or inactive disease.
ConclusionIn real world practice, AS patients under TNFi treatment show a better control of clinical symptoms than those under NSAIDs.
Evaluar si existen diferencias entre los pacientes con espondilitis anquilosante (EA) tratados con AINE o inhibidores del TNF (anti-TNF), con relación a la actividad de la enfermedad en la vida real.
MétodosEstudio observacional transversal unicéntrico con recopilación retrospectiva de datos de historias clínicas de todos los pacientes de EA examinados en nuestro hospital. Comparamos la actividad clínica, medida con la puntuación del Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), entre los pacientes tratados con AINE y los tratados con anti-TNF, en términos de baja actividad de la enfermedad definida como BASDAI<4 e inactividad, con BASDAI≤2. Como variables secundarias recopilamos también los datos clínicos, epidemiológicos y radiológicos de dichos pacientes.
ResultadosIncluimos en el estudio un total de 152 pacientes de EA (81% varones), con una edad media de 49,45±12,38años y una duración de la enfermedad de 13,5±9,79años. Ochenta y nueve pacientes (58,6%) fueron tratados con AINE y 63 (41,4%) con anti-TNF. La proporción de pacientes con baja actividad de la enfermedad e inactividad fue significativamente superior en el grupo de terapia anti-TNF, en comparación con el grupo AINE: 81 vs. 47, p=0,0001, y 44 vs. 24, p=0,007, respectivamente. Los pacientes tratados con AINE reflejaron también un dolor global significativamente mayor que aquellos con terapia de anti-TNF. La puntuación BASFI, y especialmente el tipo de tratamiento (AINE o anti-TNF), fueron las únicas variables independientemente asociadas a baja actividad de la enfermedad o a inactividad de esta.
ConclusiónEn la práctica real, los pacientes de EA con terapia anti-TNF reflejan un mejor control de los síntomas clínicos que aquellos con tratamiento de AINE.
Axial Spondyloarthritis is a group of chronic and inflammatory diseases. Ankylosing spondylitis (AS) is the most representative disease of this group and the burden of AS has recently been recognized as severe, frequently leading to invalidity, work loss and social impairment.1,2 The cornerstone of the AS treatment are non-steroidal anti-inflammatory drugs (NSAID), which have demonstrated a reduction of symptoms in about 60% of patients.3,4TNF inhibitors (TNFi) have proved to be highly effective in controlling clinical symptoms in those patients who do not respond to NSAID.5 Most of the recent recommendations of treatment suggest that a “treat to target” strategy with a tight control should be established in AS patients in order to achieve and maintain AS inactivity or low disease activity.6,7 Moreover, recent studies support that a strict control of disease activity would be essential to avoid severe disability and structural damage in AS patients8,9 independently of the treatment. In this context it could be expected that patients with AS achieved the therapeutic goal (inactive or low disease activity) despite treatment (NSAID or TNFi). However, this is not the impression we have in clinical practice. In order to asses if there are any differences in the control of disease related to the treatment prescribed, we have compared the group of patients visited in our hospital who were under NSAID treatment with those under TNFi, using the BASDAI as the main variable to assess clinical disease activity.
Method and materialsThis is an observational cross-sectional unicentric study with retrospective retrieval of data from clinical records of all patients visited as outpatients at the University Hospital of ParcTaulí that fulfilled the New York modified (NYm) criteria for AS. The study was evaluated and accepted by the local ethics commitee. We excluded patients who suffered from inflammatory bowel disease or psoriasis related to AS in order to increase the homogenicity of the sample. We also excluded those with any other associated pathology that could modify the clinical evaluation of the disease (including fibromyalgia), and those without enough data in their record to confirm the current treatment or to establish disease activity by BASDAI score.
We used the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) to measure disease activity. According to the BASDAI score we defined inactive disease (BASDAI≤2) and low disease activity (BASDAI<4).7 We also recorded in all patients included: sex, age, treatment (type and dose), disease duration (years), night back pain, patient's global score and physician's global score, all of them by visual analogous scale (VAS 0 to 10cm), the presence of extra-articular manifestations, C-Reactive Protein (CPR), Erythrocyte Sedimentation Rate (ESR), the Bath Ankylosing Spondylitis Functional Index (BASFI) and the presence or not of radiographic axial structural damage (cervical and lumbar vertebral segments). We defined the concept of vertebral ankylosis in those patients who presented a radiographic damage≥50% of the total possible damage measured by mSASSS in any segment (lumbar and/or cervical).
Statistical analysisWe performed a descriptive analysis comparing both groups of treatment (NSAID, TNFi), using Chi square test for qualitative variables and T or U test for quantitative ones, depending on their distribution. We also performed an univariate analysis in order to assess the relationship between the most relevant variables to low disease activity or inactive disease (BASDAI<4, BASDAI≤2 respectively). Also those with P<.1 were included in a multivariate model to determine if their association was independent and suppress the possible effect of confusing factors.
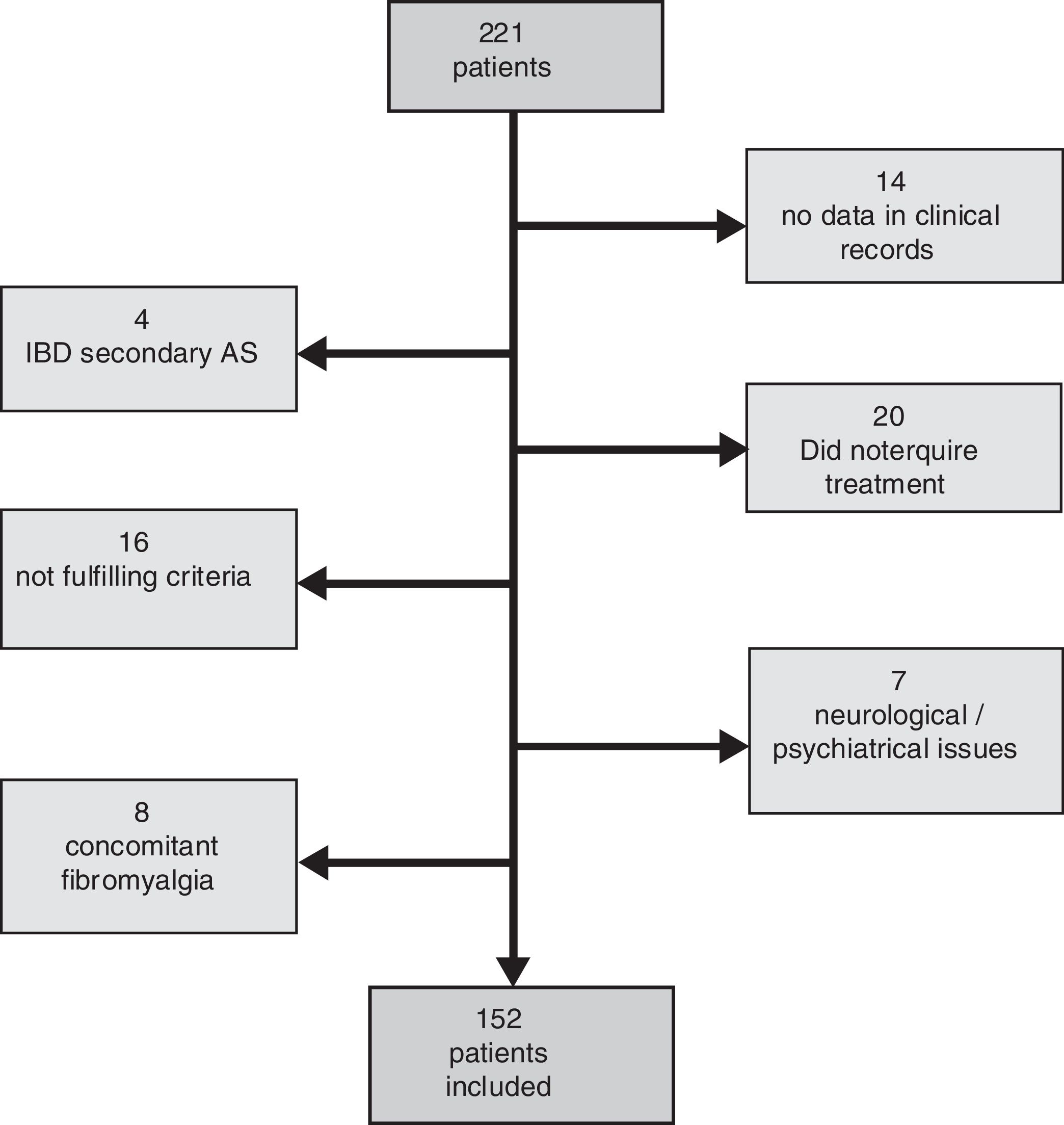
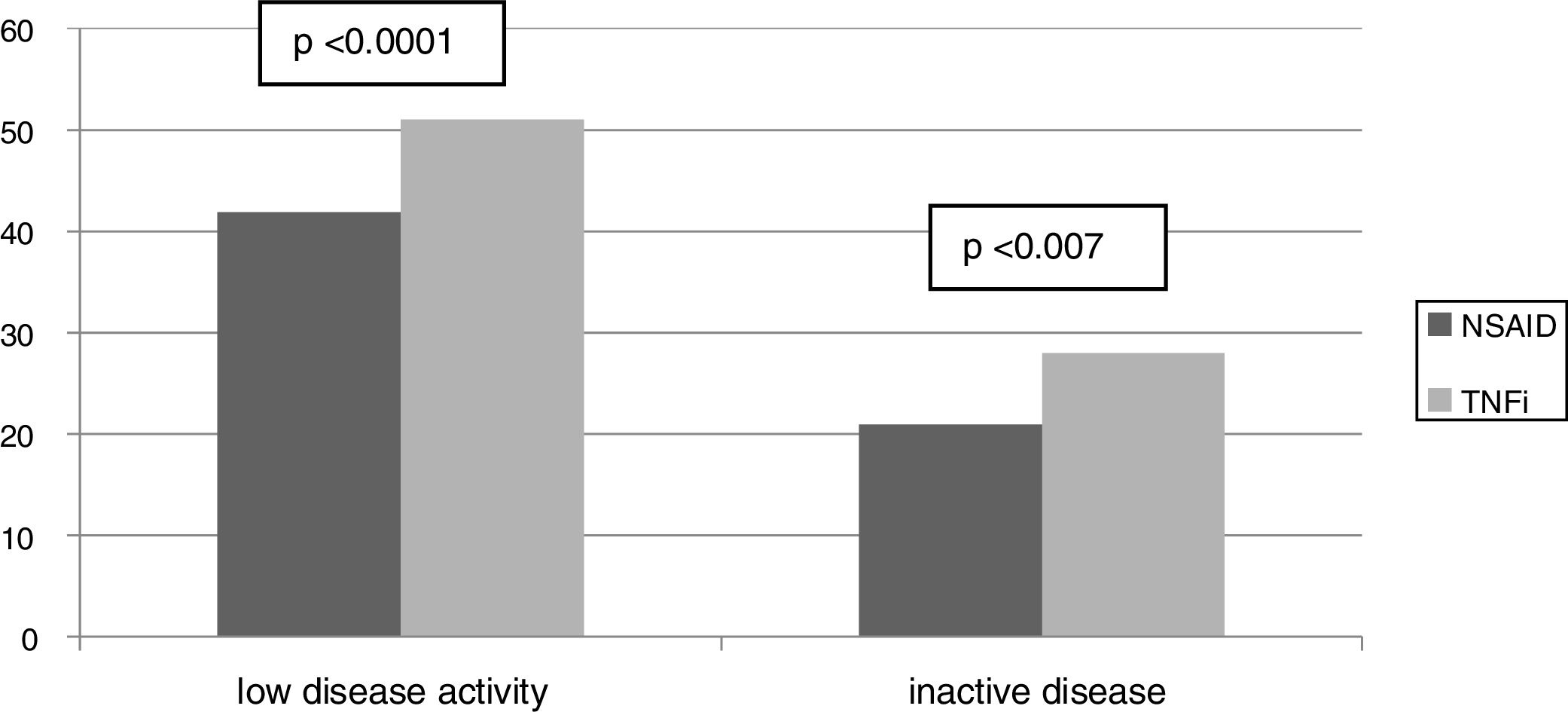
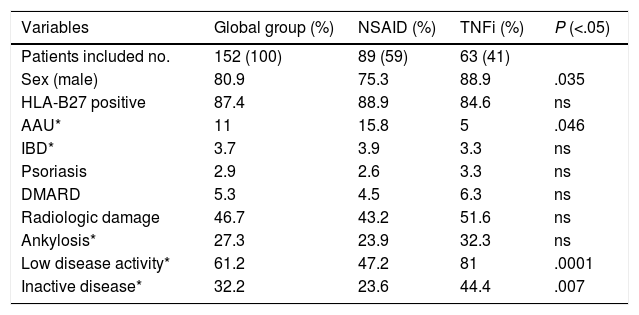
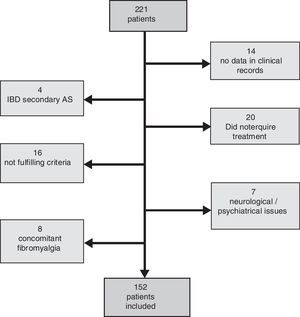
ResultsFrom the 221 patients listed in our AS database, 152 (69%) fulfilled the inclusion criteria. Patients excluded were mainly because of the presence of any concomitant disease or because of insufficient data to evaluate the BASDAI score (Fig. 1: Patients flowchart). There was a male predominance (81%) with an average age of 49.4±12.3 years and average disease duration of 13.5±9.8 years. The HLA-B27 was positive in 87% of patients. Some degree of radiologic damage was observed in 47% of patients and 17% of them showed vertebral ankylosis. The proportion of patients with low disease activity was 61%; however, only 33% of them reached an inactive disease status. Regarding the treatment prescribed, 89 patients (59%) were under NSAID (half of them on continuous treatment, 25% were taking full doses), and 63 patients (41%) were treated with TNFi (49% first, 37% second, 8% third and 6% fourth). Comparing patients according to the treatment performed (Table 1) we observed a significantly higher proportion of patients with low clinical disease activity (82 vs. 47.2%, P=.0001) and inactive disease (44.4 vs. 23.6%, P=.007) in the group of patients with TNFi treatment compared to those with NSAID (Fig. 2). Patients on NSAID treatment also showed a significantly higher proportion of female sex, anterior acute uveitis (AAU), and higher scores of BASDAI, nocturnal pain, and both patient's and physician's global assessment in comparison to those on TNFi. The disease duration was significantly higher in patients on TNFi compared with those on NSAID treatment. We did not observe any differences comparing both groups in any of the other variables analyzed, including the use of DMARD or the type of TNFi (Table 1).
Comparative analysis of clinical and demographic characteristic of patients included according to treatment (NSAID or TNFi).
| Variables | Global group (%) | NSAID (%) | TNFi (%) | P (<.05) |
|---|---|---|---|---|
| Patients included no. | 152 (100) | 89 (59) | 63 (41) | |
| Sex (male) | 80.9 | 75.3 | 88.9 | .035 |
| HLA-B27 positive | 87.4 | 88.9 | 84.6 | ns |
| AAU* | 11 | 15.8 | 5 | .046 |
| IBD* | 3.7 | 3.9 | 3.3 | ns |
| Psoriasis | 2.9 | 2.6 | 3.3 | ns |
| DMARD | 5.3 | 4.5 | 6.3 | ns |
| Radiologic damage | 46.7 | 43.2 | 51.6 | ns |
| Ankylosis* | 27.3 | 23.9 | 32.3 | ns |
| Low disease activity* | 61.2 | 47.2 | 81 | .0001 |
| Inactive disease* | 32.2 | 23.6 | 44.4 | .007 |
| Quantitative variables | Mean (SD) | Mean (SD) | Mean (SD) | P (<.05) |
|---|---|---|---|---|
| Age (years) | 49.349 (12.3799) | 49.697 (12.9496) | 48.857 (11.6118) | ns |
| BASDAI (cm) | 3.407 (2.2038) | 3.926 (2.0649) | 2.675 (1.8466) | .0001 |
| BASFI (cm) | 3.350 (2.5311) | 3.492 (2.5443) | 3.110 (2.5208) | ns |
| Night pain (VAScm) | 2.455 (2.2038) | 2.924 (2.2698) | 1.813 (1.9697) | .035 |
| Patient's global assessment (VAScm) | 3.357 (2.3310) | 3.854 (2.3632) | 2.674 (2.1344) | .022 |
| Physician's global assessment (VAScm) | 2.042 (1.6137) | 2.487 (1.7301) | 1.515 (1.3020 | .01 |
| CRP (mg/dl) | 0.8469 (1.34333) | 0.7544 (0.95489) | 0.9748 (1.7445) | ns |
| ESR | 14.97 (15.876) | 14.99 (12.618) | 14.96 (19.656) | ns |
| Disease duration (years) | 13.572 (9.7855) | 12.202 (10.0229) | 15.508 (9.1720) | .04 |
ns: non-significant; AAU: anterior acute uveitis; IBD: inflammatory bowel disease; ankylosis: radiographic damage≥50% of total possible damage measured by mSASSS in any segment (lumbar and/or cervical); low disease activity: BASDAI<4; inactive disease: BASDAI≤2; VAS: visual analogue scale; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate.
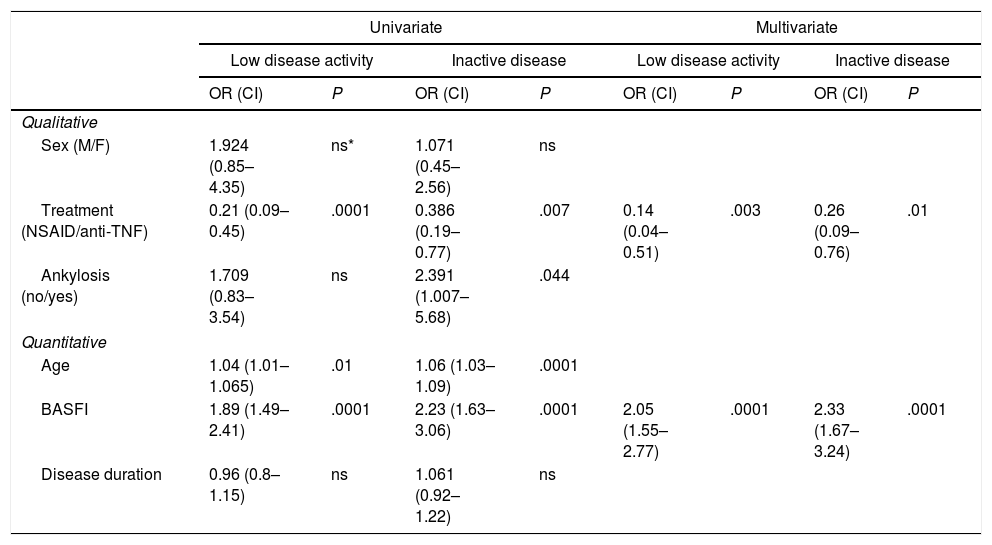
In the univariate analysis the type of treatment, age, BASFI score and the presence of vertebral ankylosis were all associated with low disease activity. In the multivariate analysis type of treatment and BASFI score were the only independent variables associated to both low disease activity and inactive disease (Table 2).
Analysis of variables associated to low disease activity or inactive disease.
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| Low disease activity | Inactive disease | Low disease activity | Inactive disease | |||||
| OR (CI) | P | OR (CI) | P | OR (CI) | P | OR (CI) | P | |
| Qualitative | ||||||||
| Sex (M/F) | 1.924 (0.85–4.35) | ns* | 1.071 (0.45–2.56) | ns | ||||
| Treatment (NSAID/anti-TNF) | 0.21 (0.09–0.45) | .0001 | 0.386 (0.19–0.77) | .007 | 0.14 (0.04–0.51) | .003 | 0.26 (0.09–0.76) | .01 |
| Ankylosis (no/yes) | 1.709 (0.83–3.54) | ns | 2.391 (1.007–5.68) | .044 | ||||
| Quantitative | ||||||||
| Age | 1.04 (1.01–1.065) | .01 | 1.06 (1.03–1.09) | .0001 | ||||
| BASFI | 1.89 (1.49–2.41) | .0001 | 2.23 (1.63–3.06) | .0001 | 2.05 (1.55–2.77) | .0001 | 2.33 (1.67–3.24) | .0001 |
| Disease duration | 0.96 (0.8–1.15) | ns | 1.061 (0.92–1.22) | ns | ||||
ns: non signifficative; low disease activity: BASDAI<4; inactive disease: BASDAI≤2.
The results we present here are not about comparing efficacy between both treatments. The results of the study highlighted the real situation in clinical practice of patients under TNFi treatment compared with those with NSAID. The study clearly demonstrated a better control of clinical symptoms in patients treated with TNFi compared to those treated only with NSAID. In this sense, not only the BASDAI score but also all the rest of clinical variables including the patient's and physician's global assessment and especially pain scores were significantly better in patients treated with TNFi compared with those only treated with NSAID.
Considering that the pain is one of the main factors related to a worse quality of life in rheumatic conditions10 it would be reasonable to establish this as one of the main goals of the AS treatment. Patients treated with TNFi were comparable to those with NSAID except for the percentage of females, which was significantly higher in the group under NSAID treatment. Some papers have reported a lower ratio of clinical response in women.11 However, it is difficult to assume this as the main explanation of the results observed. Another possible explanation could be related to the physician's opinion. Given the physician global opinion is essential to modify the previous treatment6,7 a favourable physician's opinion for patients under NSAID treatment, may explain these results but physician's global VAS in our study was clearly higher in patients under NSAID treatment compared with those on TNFi.
Biological treatments are expensive and their long-term safety remains controversial,12 and strategy of “treat to target” with a tight control has been proposed. In our clinical experience it is difficult to maintain full doses of NSAID treatment for a long time even in those patients who need them due to a moderate clinical disease activity. The fact that only half of patients took NSAID continuously and 25% of them were on full doses, clearly suggests a lower monitoring strategy in these patients. In this sense, the idea that the results we observed could be, at least partially, due to a strict tight control strategy in patients on TNFi treatment is highly suggestive, although it needs to be confirmed. We did not observe any difference in the rest of parameters analyzed between both groups of AS patients including CRP serum levels. The sample size and the low sensitivity of CRP detecting active disease in patients with a pure axial AS could explain these results.
Regarding the variables associated to good clinical response, the type of treatment, age, BASFI and presence of vertebral ankylosis were independently associated with a BASDAI<4 independently of the treatment performed, data in accordance with previous report using TNFi.13,14 In the multivariate analysis only the BASFI and especially treatment with TNFi remained as independent factors for good clinical response.
The outcome impact of the data here presented is difficult to predict. A low disease activity during follow-up measured by ASDAS-CRP was recently associated to less disability and structural radiologic damage, but the relationship between the disease activity measured by BASDAI and the vertebral radiologic progression is controversial.8 Recently, Sieper et al.15 demonstrated that a strict control of clinical disease activity measured by BASDAI, independently of the progression of radiologic vertebral damage, was associated to non-progression of patient disability; reinforcing the need to keep low BASDAI scores during follow-up.
Patients under TNFi treatment also presented significantly less episodes of AAU than those under NSAID treatment, although the type of study (retrospective and transversal) is not the best design to shed light on this subject. This data is in agreement with a better disease control of patients under TNFi treatment compared to those under NSAID.
We must underline several limitations of the study: we had a relatively high percentage of patients excluded (31.2%); however, the strict application of inclusion criteria ensured the homogeneity of the sample and the precision of the measures recorded. The sample size and the characteristics of the study performed (retrospective and cross-sectional) could make difficult to clearly establish a relationship between the parameters analyzed, clinical disease activity and especially outcomes. It would also have been interesting to record other variables that could influence the treatment received such as time since the treatment onset, comorbidities or previous treatments, in order to know why clinically active patients are only under NSAID treatment despite fulfilling biologic treatment criteria. Nevertheless, the study was not designed for this objective and further studies are needed to give response to these questions.
Despite this, the reported data clearly demonstrated in a real world life (RWL) experience a better control of AS clinical symptoms in patients treated with TNFi compared with those on NSAID. In clinical practice, patients under TNFi can take NSAID but we did not record information about NSAID intake. Finally, using BASDAI instead of ASDAS-CRP score to measure disease activity could be one of the main weaknesses of the study, however this is a retrospective pragmatical study, and the BASDAI score is still the usually performed tool to assess disease activity in AS patients. Separate scores in BASDAI, which we need to calculate ASDAS score, were not available so it could not be assessed retrospectively.
In conclusion, AS patients under TNFi treatment had a better clinical disease control than those only under NSAID.
Conflicts of interestThe authors declare no conflicts of interest.