Systemic lupus erythematosus (SLE) is characterized by a wide spectrum of clinical and immunological abnormalities. New data have emerged about the role of inflammasomes in autoimmune diseases. We aimed to investigate whether basal inflammasome activation occurs in SLE patients, and whether a relationship between inflammasome-related-cytokines and disease activity exists.
MethodsFourteen (14) consecutive SLE patients and 13 healthy individuals, matched by sex, age and ethnicity, were included. Demographics, laboratory and clinical data were recorded. Peripheral blood mononuclear cells (PBMCs) from patients and controls were obtained and monocytes were isolated by negative selection. Purified monocytes were stimulated with LPS in the presence or absence of Caspase-1 inhibitor. CD14 and Caspase-1 expression were analyzed by flow cytometry. Cytokine levels were determined in plasma and culture supernatants by ELISA. Student's t test and Mann–Whitney tests were used for statistical analysis.
ResultsThe percentage of CD14+/Caspase-1+ was significantly higher in monocytes from SLE patients compared to normal controls (p<0.01). These findings paralleled with higher plasma levels of IL-1β (p<0.05) and IL-18 (p<0.01) in those patients. Purified monocytes from SLE patients displayed a robust inflammatory response after LPS stimulation where Caspase-1, IL-1β and IL-18 were highly expressed. Plasma levels of IL-18 were also significantly higher in SLE patients with active disease (p<0.05). In addition, the production of IL-18 was reduced by 3 fold when Caspase-1 inhibitor was added to the cultures.
ConclusionsMonocytes from SLE patients exhibited increased inflammasome activation, characterized by high expression of Caspase-1, IL-1β and IL-18. Caspase-1 specific inhibitor decreased inflammasome activation (in vitro) by suppressing the production of IL-18.
El lupus eritematoso sistémico (LES) se caracteriza por presentar diversas anormalidades clínicas e inmunológicas. El ensamblaje de los componentes del inflamasoma da lugar a la activación de caspasa-1, generando la liberación de citoquinas pro-inflamatorias IL-1β e IL-18.
ObjetivosEvaluar si existe una activación basal del inflamasoma en pacientes con LES y determinar la asociación de las citoquinas IL-1β e IL-18 con la actividad de la enfermedad.
Materiales y métodosSe incluyeron 14 (n=14) pacientes consecutivos con LES y 13 (n=13) controles, pareados por edad, sexo y raza. Se recogieron datos clínicos, demográficos y de laboratorio. Los monocitos fueron aislados a partir de células mononucleares de sangre periférica obtenidas de pacientes y controles. Los monocitos purificados fueron estimulados con LPS, en presencia y ausencia de inhibidor de caspasa-1. La expresión de CD14 y caspasa-1 fueron determinados por citometría de flujo. Niveles de citoquinas fueron determinadas en plasma y en sobrenadantes de cultivos mediante técnica de ELISA. Test de Student y Mann-Whitney fueron usados para el análisis estadístico.
ResultadosEl porcentaje de CD14+/caspasa-1+ fue significativamente superior en monocitos de pacientes con LES vs. controles (p<0,01). En forma paralela, se encontraron niveles plasmáticos significativamente superiores de IL-1β (p<0,05) y de IL-18 (p<0,01) en pacientes con LES. Monocitos purificados de pacientes lúpicos presentaron una robusta respuesta inflamatoria luego de ser estimulados con LPS, donde caspasa-1, IL-1β e IL-18 fueron altamente expresados. Niveles plasmáticos de IL-18 fueron significativamente mayores en pacientes con LES y enfermedad activa (p<0,05). Por otro lado, la producción de IL-18 se redujo casi 3 veces cuando se agregó inhibidor de caspasa-1 en cultivos.
ConclusionesMonocitos de pacientes con LES presentaron evidencia de activación de componentes del inflamasoma, caracterizada por una mayor expresión de caspasa-1, IL-1β e IL-18. El inhibidor específico de caspasa-1 disminuyó la activación del inflamasoma in vitro, reduciendo la producción de IL-18.
Systemic lupus erythematosus (SLE) is a prototype of autoimmune disease, characterized by a wide spectrum of clinical and immunologic abnormalities.1
Exciting data have emerged over the last few years, regarding the role of the inflammasome as an important component of the innate immune system.2–4
The inflammasome is a large molecular platform constituted by a group of cytoplasmic protein complexes that senses a diverse set of inflammation-inducing stimuli, including pathogen-associated molecular patterns (PAMPs), predominantly found in microbes and damage-associated molecular patterns (DAMPs) that are released as a result of perturbations of tissue homeostasis caused by microbial or non-microbial insults. The central components of the inflammasome include: (1) scaffold of the NOD-like receptors (NLR) family: NLRP1, NLRP3, NLRC4 and AIM2; (2) apoptosis associated speck-like protein containing a CARD (ASC); and (3) pro-Caspase-1. The activated sensor can then recruit the other components of the inflammasome, leading to the formation of the multiprotein complex containing pro-Caspase-1. The assembly of these components leads to the autoproteolytic activation of Caspase-1, which converts an immature IL-1β and IL-18 to a mature secreted form.5–7
Because the precise etiology and mechanisms leading to aberrant immune responses in SLE are not yet clearly understood, we hypothesized that: “inflammasome activation occurs in monocytes, as a key element on the initiation and amplification of the innate immune response in SLE pathogenesis”. Therefore, the aims of this study were: (1) To determine whether inflammasome activation occurs in monocytes of SLE patients; and (2) to determine the relationship between the production of inflammasome related-cytokines and disease activity in these patients.
Materials and methodsPatients selectionConsecutive patients (n=14) who fulfilled the revised ACR criteria for SLE were enrolled at Louisiana State University (LSU) outpatient Rheumatology clinics. Consecutive patients without any inflammatory rheumatic disease were included as healthy controls (n=13), matched by age, sex and ethnicity. Patients with acute and/or chronic infections, including history of positive serology to HIV, HBV and HCV were excluded. All participants signed an informed consent form prior to their inclusion, which was approved by LSU Institutional Review Board.
Demographic, laboratory, and clinical data were recorded. Lupus disease activity was assessed by SELENA-SLEDAI score.8 A score ≥4 was defined as active disease. Lupus nephritis (LN) (absent, past, current) was also recorded.
Collection and processing of blood samplesPeripheral blood mononuclear cells (PBMCs) from patients and controls were isolated by Ficoll density gradient centrifugation (Sigma Chemicals Co., St Louis, MO, USA). CD14+ monocytes were purified from PBMCs, using an autoMACS Separator and an autoMACS CD14+ negative selection kit (Miltenyi Biotec, Germany). Purified monocytes were cultured in six well plates (1×106monocytes/well) in RPMI 1640, supplemented with 10% fetal bovine serum, 10mM HEPES and 2mM l-Glutamine. After cell adherence, monocytes were stimulated (∼18h) with lipopolysaccharide (LPS) (100ng/ml) in presence or absence of Caspase-1 inhibitor (10μM) (Caspase-1 inhibitor I, cell-permeable, YVAD-CHO) (Calbiochem, Germany). The remaining supernatants were stored at −70°C until assayed.
Intracellular Caspase-1 detectionFresh PBMCs were fixed-permeabilized, and stained with CD14+ (FITC) (Beckman Coulter, USA) and Caspase-1 (A-19) (PE) antibodies (Santa Cruz Biotechnology, USA). Caspase-1 expression was measured by flow cytometry on a BD FACSCantor II flow cytometer (Becton Dickinson, San Jose, CA). Log fluorescence of the gated population was measured and 20,000 cells were acquired for each sample. Caspase-1 expression corresponded to the average of 2 determinations made for each patient and control. Data analysis was performed using BD FACSDiva software version 6.1.3.
Cytokine measurementIL-1β and IL-18 levels were determined in patients plasma and cell culture supernatants by human Quantikine ELISA kits (R&D Systems, USA), following the manufacturer's instructions. Since the assay range for IL-1β is between 3.9 and 250pg/ml, we extended down the lower range of the standards 3 dilutions giving us a range between 0.100–250pg/ml to maximize the presence of low values in our samples. These extra dilutions did not alter the linearity of the standard curve. Plasmatic cytokine levels of IL-1beta and IL-18 corresponded to the average of 2 determinations made for each patient and control.
Statistical analysisDescriptive values of variables were expressed as the mean±SD. Variables with normal distribution were analyzed by Student's t-test and those with non-normal distribution were analyzed with Mann–Whitney test. p-Values less than 0.05 were considered significant. The statistical analysis was carried out using GraphPad Prism software (version 5.0).
ResultsFourteen (14) consecutive SLE patients were included in the study. Thirteen out of 14 patients (93%) were female and 8/14 (57%) were African-Americans. The mean age of the patients at the time of the study was 32.7 (±9.1) years, and the mean disease duration was 8.9 years (±6.4).
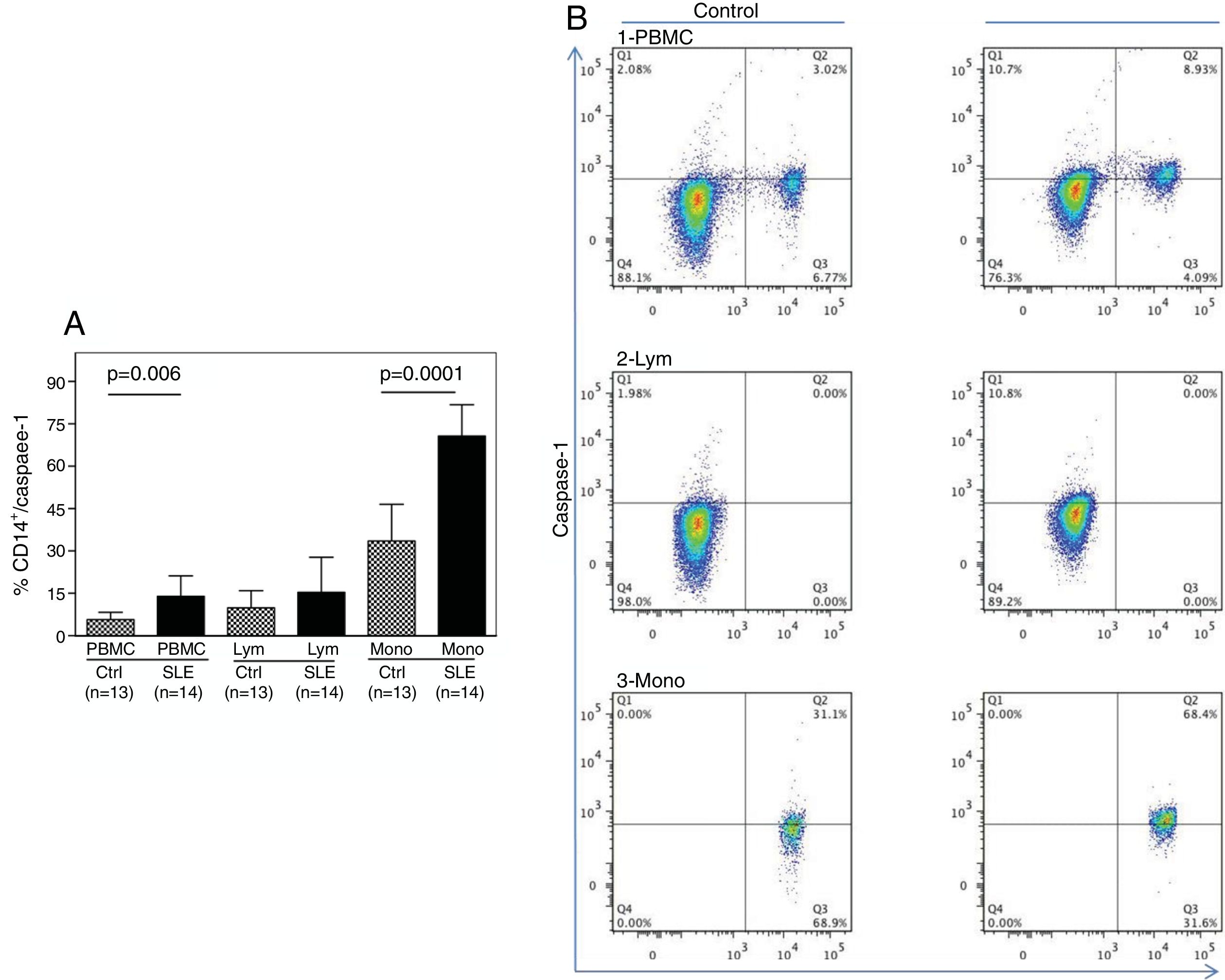
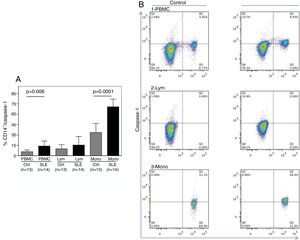
As shown in Fig. 1A, the percentage of CD14+ vs. Caspase-1+ expression was found to be significantly higher in fresh PBMCs and in the monocytic population from SLE patients compared to normal controls (p=0.006 and p<0.0001, respectively). Fig. 1B shows the percentage of Caspase-1 positive cells in PBMCs and monocytes (quadrant 2) from one control and one SLE-patient. The majority of Caspase-1 positive cells were observed in the monocytic population from patients with SLE (68.4%).
Percentage of CD14+/Caspase-1+ expressing-cells. A. Cells were gated using FS vs SS to determine the % of CD14+/Caspase-1+ cells in PBMC and Mono. For lymphocytes it is only Caspase-1+. Data from 14 SLE patients and 13 normal controls are presented, as the mean±SD. B. Representative data from 1 normal control and 1 SLE patient dot plot showing the expression of CD14+ vs. Caspase-1+ in PBMC (1), Lymph (2) and Mono (3). Stained cells were gated based on FSC and SSC parameters (capase-1+ lymphocytes in Q1 and PMBC and monocytes in Q2). PBMC: peripheral blood mononuclear cells; Lym: lymphocytes; Mono: monocytes.
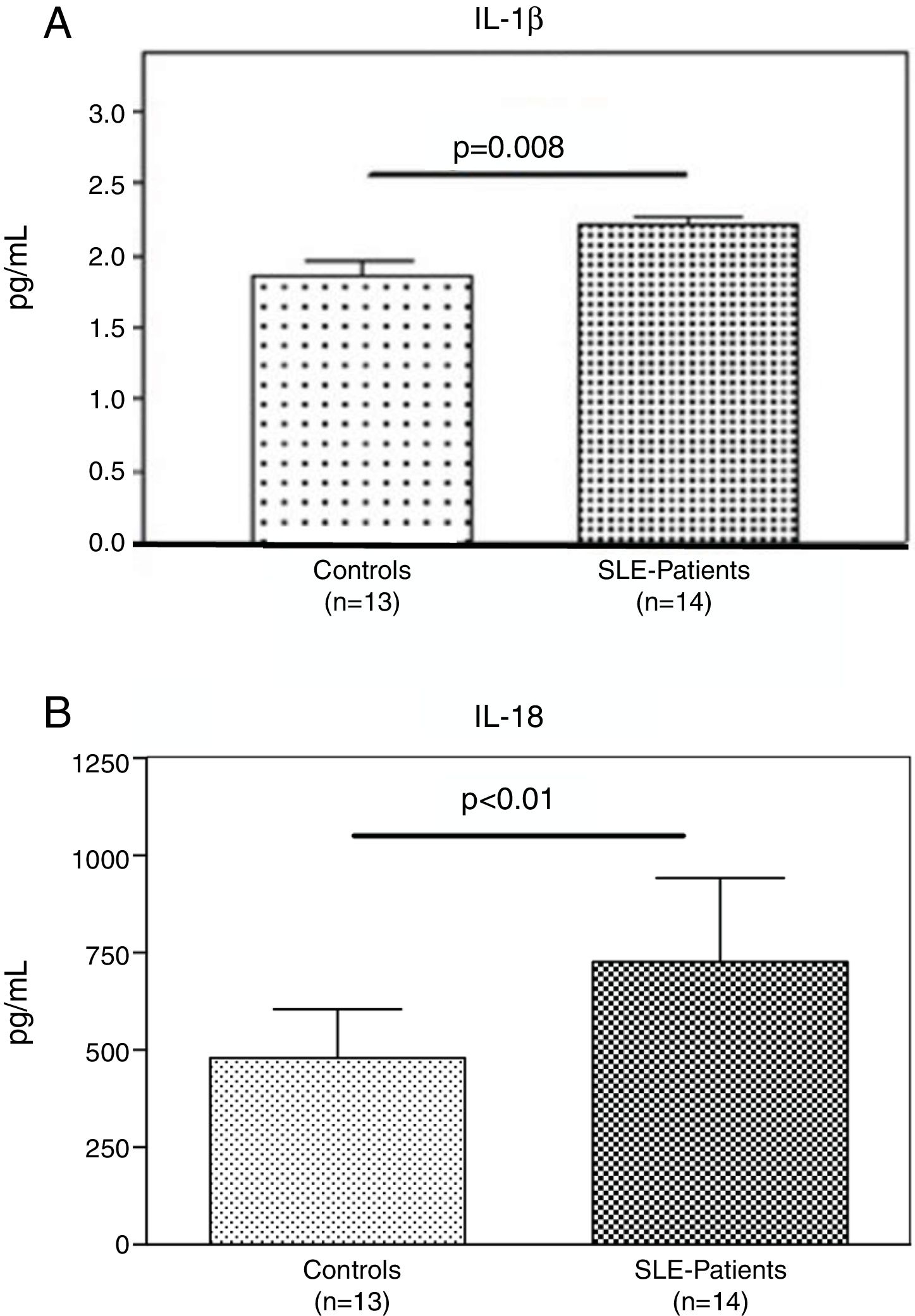
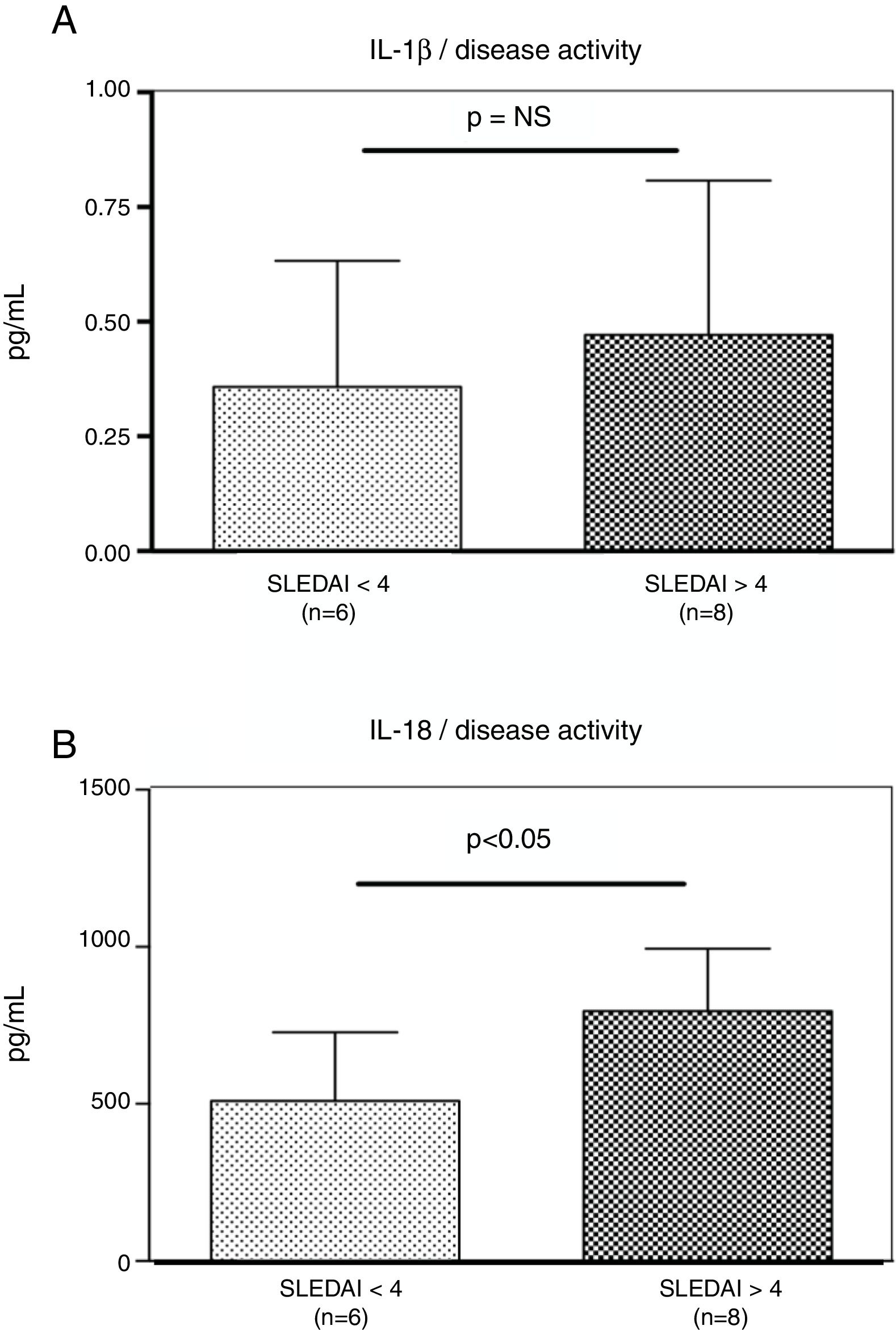
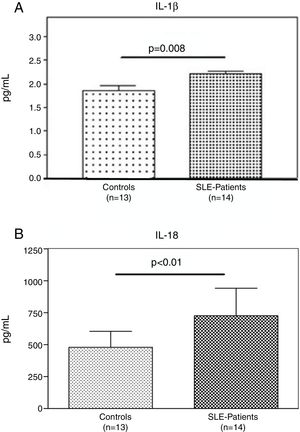
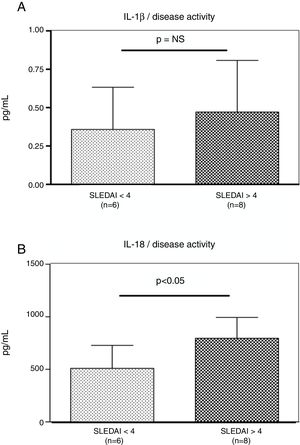
Interestingly, the increased numbers of Caspase-1 positive cells directly paralleled with significantly higher plasma levels of IL-1β (2.2±0.05pg/ml vs. 1.8±0.10pg/ml) (p=0.008) (Fig. 2A) and IL-18 (725.2±215.4pg/ml vs. 479.2±125.2pg/ml) (p<0.01) in SLE patients when compared to normal controls (Fig. 2B). Furthermore, we observed that levels of IL-18 were significantly higher (834.3±179.3pg/ml) in patients presenting high disease activity (SELENA-SLEDAI ≥6), compared to patients with mild disease or remission (572.5±171.6pg/ml) (p<0.05). No statistical differences in levels of IL-1β were observed between SLE patients with high vs. mild disease activity (Fig. 3A and B).
Plasma levels of inflammasome-related cytokines. A and B. IL-1β and IL-18 level in the plasma of (n=13) normal controls and (n=14) SLE patients were determined by ELISA. IL1β and IL-18 were significantly high expressed in SLE patients compared to normal controls. Data are presented as mean±SD from duplicates.
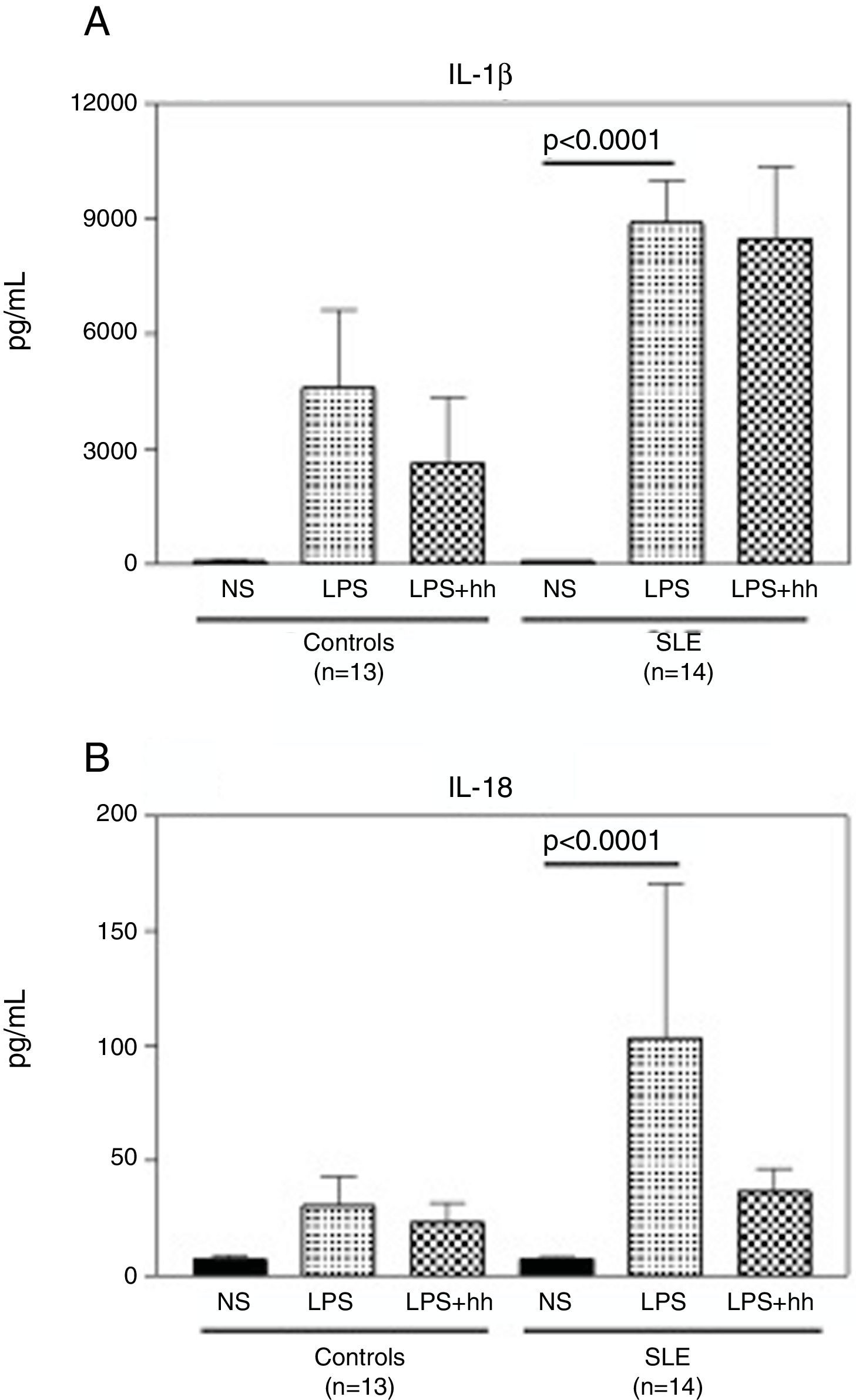
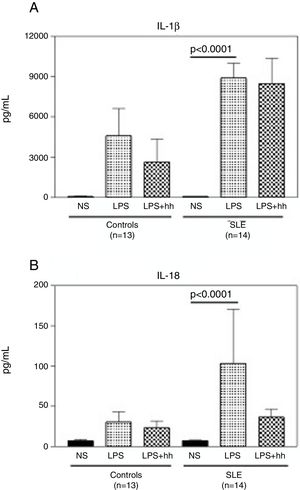
Compared to normal controls, purified monocytes from SLE patients displayed a robust inflammatory response when cultured in vitro. As shown in Fig. 4A, when monocytes from normal controls and SLE patients were stimulated with LPS, there was a 60 (mean: 4597±3515pg/ml) and 100 (mean: 8895±3475pg/ml) fold increase in IL-1β compared to non-stimulated monocytes (mean: 88.3±6.8). No significant differences in IL1-β production were observed between LPS-stimulated monocytes from controls and SLE patients. Similar production pattern of IL-18 was observed (Fig. 4B), where the mean of IL-18 production was significantly higher (p<0.0001) in supernatants from stimulated monocytes from SLE-patients (p<0.0001) than in non-stimulated. Furthermore, IL-18 production in LPS-stimulated monocytes from SLE patients was 3 fold higher when compared to normal controls (mean: 103.4±213.2pg/ml and 30.3±21.9pg/ml respectively). In Fig. 4A, there was a slight reduction in IL-1β production in LPS-stimulated normal monocytes plus the inhibitor, that was not observed in SLE patients. In contrast, as seen in Fig. 4B, a reduction of approximately 3 fold in IL-18 production was observed in supernatants of LPS-stimulated monocytes from SLE patients plus inhibitor. Significant correlations between Caspase-1 expression/cytokine production and clinical manifestations, treatment or serologic markers of the disease were not found in this study.
LPS induces secretion of high levels of IL-1β and IL-18 in monocytes from SLE patients. Purified CD14+ monocytes were incubated with LPS (100ng/ml), in the presence or absence of Caspase-1 inhibitor (10μM). IL-1β (A) and IL-18 (B) concentrations were measured in supernatants by specific ELISA after ∼18-h incubation. Data is representative as mean±SD from duplicates. NS: Non-stimulated; LPS: lipopolysaccharide; Inh: Caspase-1 inhibitor; SLE: systemic lupus erythematosus.
The major finding of this pilot study is that in vitro, monocytes from SLE patients showed an increased expression of Caspase-1 that parallels with significant higher levels of inflammasome-related cytokines, IL-1β and IL-18, in patients with SLE as compared to healthy subjects.
How the inflammasome is triggered and activated in SLE, has become an important concept to understand its role in this disease. Immune complexes formed secondary to antibody recognition of DNA or RNA antigens, have been shown to stimulate inflammasome activation through up-regulation of TLR-dependent activation of NFκβ and subsequent activation of the NLRP3 inflammasome.9,10 C3a, which is released during complement activation in tissues, promotes inflammasome activation through up-regulation of ATP secretion.11 Neutrophil extracellular traps (NETs) have also been proposed to play a pathogenic role in SLE. Recently, NETs have been shown to activate caspase-1, resulting in release of IL-1β and IL-18, and this activation was enhanced in macrophages derived from SLE patients.12
Because innate immune cells are crucial for the onset of the immune response, inflammasome activation in these cells may promote the initiation and amplification of the autoimmune response. In this study, we found an increased expression of Caspase-1 in monocytes from SLE patients, as compared with healthy controls (p<0.0001). To our knowledge this is the first study in demonstrating that basal inflammasome activation occurs in fresh isolated monocytes from SLE patients.
IL-1β and IL-18 cytokines, hallmarks of inflammasome activation, belong to the IL-1 cytokine family. IL-1β is critical to the pathology of most autoinflammatory and many autoimmune diseases. IL-18 was discovered initially as an IFN-γ inducing factor, and since then has been known as a prototypical Th-1 cytokine.13 In our study, plasma levels of these cytokines were found to be significantly higher in SLE patients as compared to normal controls. Recently, Liu et al. have also shown an enhanced inflammasome activity in SLE patients, mediated by type I IFN.14
In this study, we focused on investigating the capacity of monocytes from SLE patients to secrete inflammasome-related cytokines at the basal level, and in response to the inflammatory stimuli LPS, as compared with monocytes from healthy individuals. As expected, monocytes from SLE patients displayed a robust inflammatory response after LPS stimulation, characterized by elevated levels of IL-1β and IL-18 when compared to stimulated monocytes from healthy individuals. In addition, the production of IL-18 was reduced by almost 3 fold when Caspase-1 inhibitor was added to the cultures. There was also a reduction in levels of IL-1β in supernatants treated with Caspase-1 inhibitor, although in less extent compared to IL-18. A possible explanation might be related by the extraordinary high amount of IL-1β released, as compared to IL-18. The inhibitor is competitive, so inhibition could be overcome by substrate excess.
A growing body of evidence supports the pivotal role of infections in the induction or exacerbation of SLE. Infections can be responsible for aberrant immune response leading to a loss of tolerance toward native proteins.15 Our findings suggest that monocytes from SLE patients might have an abnormal expression of IL-18 and IL-1β-regulating molecules in response to infection, thus resulting in anomalous activation of adaptive immune responses, which can be the basis by which SLE develops and flares. On the other hand, we also agree that the main limitation of the study is the small number of the sample. However, despite this, we were able to demonstrate the activation of the inflammasome in monocytes of SLE patients.
In conclusion, our study shows that monocytes from SLE patients exhibit an enhanced activation of the inflammasome complex in vitro, which is the product of elevated expression of Caspase-1 and inflammasome-related cytokines. The results of this proof of concept study indicate a potential role of the inflammasome pathway in the pathogenesis of SLE, thus identifying a novel pathway for therapeutic targeting.
Ethical approvalAll procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consentInformed consent was obtained from all individual participants included in the study.
Conflict of interestThe authors declare that they have no conflict of interest to disclose.
We thank Cynthia Brown for assisting in the collection of blood samples.