In order to agree on the fundamental aspects related to the management of pregnancy in patients with systemic lupus erythematosus (SLE) and antiphospholipid syndrome (APS), the Spanish Societies of Gynaecology and Obstetrics, Internal Medicine and Rheumatology have set up a working group for the preparation of three consensus documents.
MethodsEach of the Scientific Societies involved proposed five representatives based on their experience in the field of pregnancy control in patients with autoimmune diseases. The recommendations were developed following the Delphi methodology.
ResultsThis third document contains the recommendations regarding the management of delivery, puerperium and lactation, including medication use during these periods and the care of the newborn. In addition, a section on contraception is included.
ConclusionsThese multidisciplinary recommendations are considered decision-making tools for clinicians involved in the care of patients with SLE/APS during pregnancy.
Las Sociedades Españolas de Ginecología y Obstetricia, de Medicina Interna y de Reumatología han constituido un grupo de trabajo paritario para la elaboración de tres documentos de consenso sobre el control del embarazo en mujeres con lupus eritematoso sistémico (LES) y síndrome antifosfolípido (SAF).
MétodosCada una de las sociedades científicas implicadas propuso cinco representantes en base a su experiencia en el área del control del embarazo en pacientes con enfermedades autoinmunes. Las recomendaciones se elaboraron siguiendo la metodología Delphi.
ResultadosEn este tercer documento se incluyen las recomendaciones que abordan el manejo parto, puerperio y lactancia, incluyendo el manejo de los diferentes fármacos en estos periodos. Además se incluye una sección sobre los cuidados iniciales del recién nacido y sobre anticoncepción.
ConclusionesEstas recomendaciones multidisciplinares se consideran herramientas en la toma de decisiones para los clínicos involucrados en la asistencia a pacientes con LES/SAF durante el embarazo.
Following on from the first two parts, devoted to preconception assessment, infertility and ovarian preservation and patient management from conception to delivery, this third section deals with the management of childbirth, puerperium, breastfeeding and contraception in women with systemic lupus erythematosus and antiphospholipid syndrome.
Material and methodsAs in the previous two parts, we used the Delph methodology to establish the recommendations. The specific working group for this section included one representative from the Spanish Society of Gynaecology and Obstetrics, one from the Systemic Autoimmune Diseases Group of the Spanish Society of Internal Medicine and one from the Systemic Autoimmune Diseases Working Group of the Spanish Society of Rheumatology, and a coordinator.
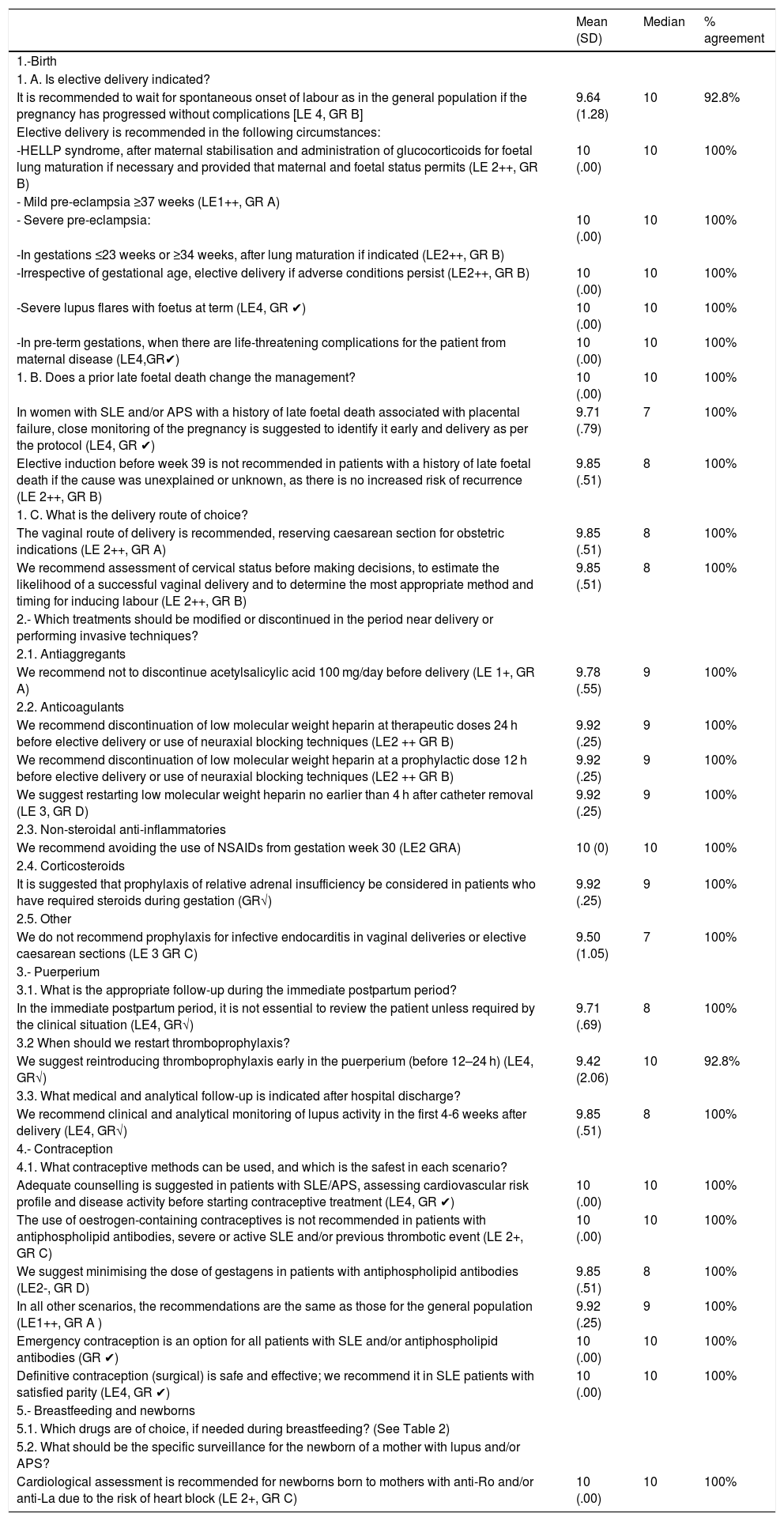
Final consensus documentThe final recommendations are shown in Tables 1 and 2.
Summary of recommendations.
| Mean (SD) | Median | % agreement | |
|---|---|---|---|
| 1.-Birth | |||
| 1. A. Is elective delivery indicated? | |||
| It is recommended to wait for spontaneous onset of labour as in the general population if the pregnancy has progressed without complications [LE 4, GR B] | 9.64 (1.28) | 10 | 92.8% |
| Elective delivery is recommended in the following circumstances: | |||
| -HELLP syndrome, after maternal stabilisation and administration of glucocorticoids for foetal lung maturation if necessary and provided that maternal and foetal status permits (LE 2++, GR B) | 10 (.00) | 10 | 100% |
| - Mild pre-eclampsia ≥37 weeks (LE1++, GR A) | |||
| - Severe pre-eclampsia: | 10 (.00) | 10 | 100% |
| -In gestations ≤23 weeks or ≥34 weeks, after lung maturation if indicated (LE2++, GR B) | |||
| -Irrespective of gestational age, elective delivery if adverse conditions persist (LE2++, GR B) | 10 (.00) | 10 | 100% |
| -Severe lupus flares with foetus at term (LE4, GR ✔) | 10 (.00) | 10 | 100% |
| -In pre-term gestations, when there are life-threatening complications for the patient from maternal disease (LE4,GR✔) | 10 (.00) | 10 | 100% |
| 1. B. Does a prior late foetal death change the management? | 10 (.00) | 10 | 100% |
| In women with SLE and/or APS with a history of late foetal death associated with placental failure, close monitoring of the pregnancy is suggested to identify it early and delivery as per the protocol (LE4, GR ✔) | 9.71 (.79) | 7 | 100% |
| Elective induction before week 39 is not recommended in patients with a history of late foetal death if the cause was unexplained or unknown, as there is no increased risk of recurrence (LE 2++, GR B) | 9.85 (.51) | 8 | 100% |
| 1. C. What is the delivery route of choice? | |||
| The vaginal route of delivery is recommended, reserving caesarean section for obstetric indications (LE 2++, GR A) | 9.85 (.51) | 8 | 100% |
| We recommend assessment of cervical status before making decisions, to estimate the likelihood of a successful vaginal delivery and to determine the most appropriate method and timing for inducing labour (LE 2++, GR B) | 9.85 (.51) | 8 | 100% |
| 2.- Which treatments should be modified or discontinued in the period near delivery or performing invasive techniques? | |||
| 2.1. Antiaggregants | |||
| We recommend not to discontinue acetylsalicylic acid 100 mg/day before delivery (LE 1+, GR A) | 9.78 (.55) | 9 | 100% |
| 2.2. Anticoagulants | |||
| We recommend discontinuation of low molecular weight heparin at therapeutic doses 24 h before elective delivery or use of neuraxial blocking techniques (LE2 ++ GR B) | 9.92 (.25) | 9 | 100% |
| We recommend discontinuation of low molecular weight heparin at a prophylactic dose 12 h before elective delivery or use of neuraxial blocking techniques (LE2 ++ GR B) | 9.92 (.25) | 9 | 100% |
| We suggest restarting low molecular weight heparin no earlier than 4 h after catheter removal (LE 3, GR D) | 9.92 (.25) | 9 | 100% |
| 2.3. Non-steroidal anti-inflammatories | |||
| We recommend avoiding the use of NSAIDs from gestation week 30 (LE2 GRA) | 10 (0) | 10 | 100% |
| 2.4. Corticosteroids | |||
| It is suggested that prophylaxis of relative adrenal insufficiency be considered in patients who have required steroids during gestation (GR√) | 9.92 (.25) | 9 | 100% |
| 2.5. Other | |||
| We do not recommend prophylaxis for infective endocarditis in vaginal deliveries or elective caesarean sections (LE 3 GR C) | 9.50 (1.05) | 7 | 100% |
| 3.- Puerperium | |||
| 3.1. What is the appropriate follow-up during the immediate postpartum period? | |||
| In the immediate postpartum period, it is not essential to review the patient unless required by the clinical situation (LE4, GR√) | 9.71 (.69) | 8 | 100% |
| 3.2 When should we restart thromboprophylaxis? | |||
| We suggest reintroducing thromboprophylaxis early in the puerperium (before 12–24 h) (LE4, GR√) | 9.42 (2.06) | 10 | 92.8% |
| 3.3. What medical and analytical follow-up is indicated after hospital discharge? | |||
| We recommend clinical and analytical monitoring of lupus activity in the first 4-6 weeks after delivery (LE4, GR√) | 9.85 (.51) | 8 | 100% |
| 4.- Contraception | |||
| 4.1. What contraceptive methods can be used, and which is the safest in each scenario? | |||
| Adequate counselling is suggested in patients with SLE/APS, assessing cardiovascular risk profile and disease activity before starting contraceptive treatment (LE4, GR ✔) | 10 (.00) | 10 | 100% |
| The use of oestrogen-containing contraceptives is not recommended in patients with antiphospholipid antibodies, severe or active SLE and/or previous thrombotic event (LE 2+, GR C) | 10 (.00) | 10 | 100% |
| We suggest minimising the dose of gestagens in patients with antiphospholipid antibodies (LE2-, GR D) | 9.85 (.51) | 8 | 100% |
| In all other scenarios, the recommendations are the same as those for the general population (LE1++, GR A ) | 9.92 (.25) | 9 | 100% |
| Emergency contraception is an option for all patients with SLE and/or antiphospholipid antibodies (GR ✔) | 10 (.00) | 10 | 100% |
| Definitive contraception (surgical) is safe and effective; we recommend it in SLE patients with satisfied parity (LE4, GR ✔) | 10 (.00) | 10 | 100% |
| 5.- Breastfeeding and newborns | |||
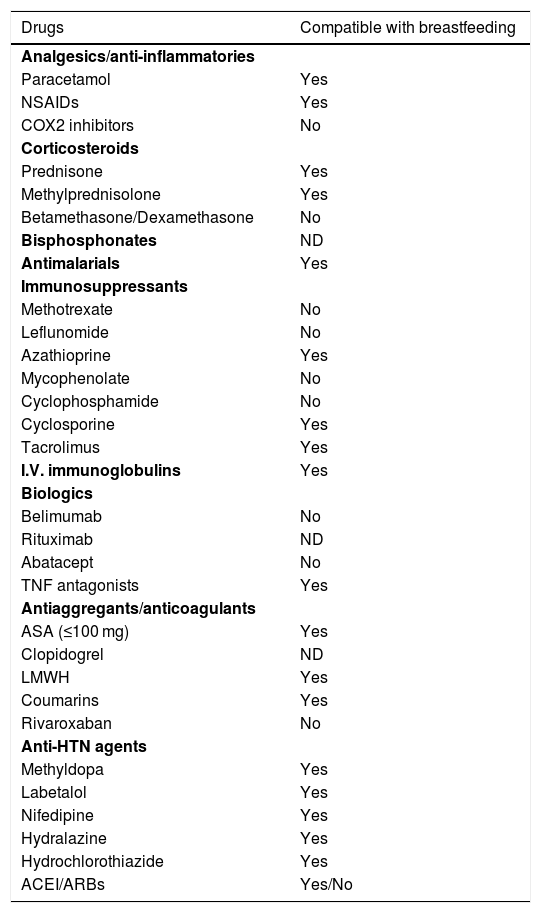
| 5.1. Which drugs are of choice, if needed during breastfeeding? (See Table 2) | |||
| 5.2. What should be the specific surveillance for the newborn of a mother with lupus and/or APS? | |||
| Cardiological assessment is recommended for newborns born to mothers with anti-Ro and/or anti-La due to the risk of heart block (LE 2+, GR C) | 10 (.00) | 10 | 100% |
Drugs and breastfeeding.
| Drugs | Compatible with breastfeeding |
|---|---|
| Analgesics/anti-inflammatories | |
| Paracetamol | Yes |
| NSAIDs | Yes |
| COX2 inhibitors | No |
| Corticosteroids | |
| Prednisone | Yes |
| Methylprednisolone | Yes |
| Betamethasone/Dexamethasone | No |
| Bisphosphonates | ND |
| Antimalarials | Yes |
| Immunosuppressants | |
| Methotrexate | No |
| Leflunomide | No |
| Azathioprine | Yes |
| Mycophenolate | No |
| Cyclophosphamide | No |
| Cyclosporine | Yes |
| Tacrolimus | Yes |
| I.V. immunoglobulins | Yes |
| Biologics | |
| Belimumab | No |
| Rituximab | ND |
| Abatacept | No |
| TNF antagonists | Yes |
| Antiaggregants/anticoagulants | |
| ASA (≤100 mg) | Yes |
| Clopidogrel | ND |
| LMWH | Yes |
| Coumarins | Yes |
| Rivaroxaban | No |
| Anti-HTN agents | |
| Methyldopa | Yes |
| Labetalol | Yes |
| Nifedipine | Yes |
| Hydralazine | Yes |
| Hydrochlorothiazide | Yes |
| ACEI/ARBs | Yes/No |
ACEI: Angiotensin-Converting Enzyme Inhibitors; ASA: Acetylsalicylic acid at Antiplatelet dose; ARBs: Angiotensin Receptor Blockers; COX2: Cyclooxygenase-2; HT: Hypertension; LMWH: Low Molecular Weight Heparin; NA: No data; NSAIDs: Non-Steroidal Anti-inflammatory Drugs; TNF: Tumour Necrosis Factor.
E. Rodríguez Almaraz has received funding for presentations, courses, and conferences from: Novartis, Roche, GSK, Menarini, Grünenthal, Abbvie, UCB and Lilly and MSD research grants.
V. Martínez-Taboada has received funding from Roche for an independent research project, and from Sanofi for presentations.
The remaining authors have no conflict of interests to declare.
Please cite this article as: Delgado P, Robles Á, Martínez López JA, Sáez-Comet L, Rodríguez Almaraz E, Martínez-Sánchez N, et al. Manejo del embarazo en pacientes con lupus eritematoso sistémico/síndrome antifosfolípido. Parte 3. Parto. Puerperio. Lactancia. Anticoncepción. Recién nacido. Reumatol Clin. 2021;17:183–186.
Consensus document of the Spanish Society of Gynaecology and Obstetrics (SEGO), Spanish Society of Internal Medicine (SEMI) and Spanish Society of Rheumatology (SER). The full text is available as additional material in the Annex.