The objective was to assess the influence of patients’ weight in the cost of rheumatoid arthritis treatment with biologic drugs used in first line after non-adequate response to methotrexate.
Patients and methodPharmaceutical and administration costs were calculated in two scenarios: non-optimization and optimization of intravenous (IV) vials. The retrospective analysis of 66 patients from a Spanish 1000 beds-hospital Rheumatology Clinic Service was used to obtain posology and weight data. The study time horizon was two years. Costs were expressed in 2013 euros.
ResultsFor an average 69kg-weighted patient the lowest cost corresponded to abatacept subcutaneous (SC ABA) (€21,028.09) in the scenario without IV vials optimization and infliximab (IFX) (€20,779.29) with optimization. Considering patients’ weight in the scenario without IV vials optimization infliximab (IFX) was the least expensive drug in patients ranged 45–49kg, IV ABA in 50–59kg and SC ABA in patients over 60kg. With IV vials optimization IFX was the least expensive drug in patients under 69kg and SC ABA over 70kg.
ConclusionsAssuming comparable effectiveness of biological drugs, patient's weight is a variable to consider, potentials savings could reach €20,000 in two years.
Evaluar la influencia del peso de los pacientes en el coste de tratamiento de la artritis reumatoide con fármacos biológicos indicados en primera línea tras respuesta inadecuada a metotrexato.
Pacientes y métodoSe incluyeron costes farmacológicos y de administración considerando no optimización y optimización de viales de los fármacos intravenosos. Los datos de posologías y peso de pacientes se obtuvieron de forma retrospectiva de 66 pacientes atendidos en un servicio de Reumatología Clínica de un hospital terciario en España. El horizonte temporal del estudio fue de 2 años. Los costes se expresaron en euros de 2013.
ResultadosEn un paciente promedio de 69 kg de peso, abatacept subcutáneo (ABA SC) fue el fármaco de menor coste (21.028,09 €) sin optimización de viales, e infliximab (IFX) (20.779,29 €) con optimización de viales. Considerando el peso de los pacientes, sin optimización de viales, IFX fue menos costoso en pacientes de 45-49 kg, ABA intravenoso en pacientes de 50-60 kg y ABA SC en pacientes ≥ 60 kg. Con optimización de viales IFX sería menos costoso en pacientes < 70 kg y ABA SC pacientes ≥ 70 kg.
ConclusionesSuponiendo efectividad comparable de fármacos biológicos, el peso de los pacientes es una variable relevante, pudiendo alcanzarse ahorros potenciales superiores a 20.000 € en pacientes de más de 100 kg de peso en 2 años de tratamiento.
Rheumatoid arthritis (RA) has a great impact on patient quality of life, and the social and economic costs are elevated.1 It has a negative effect on the morbidity and disability of the patients,2 who see their life expectancy reduced by 5–10 years,3 and severely affects their activities of daily living.4,5
There have been important advances in its treatment, due in part to disease-modifying antirheumatic drugs (DMARDs), which are the treatment of choice in RA.6 After a first-line treatment with conventional DMARDs like methotrexate (MTX), the next therapeutic option is comprised of the so-called biologic DMARDs, produced in cell culture using genetic engineering.7
In Spain, after an inadequate response to MTX, first-line treatment includes subcutaneous abatacept (SC ABA), intravenous abatacept (IV ABA), adalimumab (ADA), certolizumab (CZP), etanercept (ETN), golimumab (GLM), infliximab (IFX), tocilizumab (TCZ) and anakinra. Although there are a few studies that directly compare any of these agents, such as the AMPLE8 and ADACTA9 trials, much of the available information comes from indirect comparisons, which show slight differences in terms of safety or efficacy.10
The doses of those biologic agents that are administered IV (IFX, ABA and TCZ) are adjusted according to patient weight. The remainder, including SC ABA, are administered subcutaneously at fixed doses, independent of body weight. The main objective of this study was to evaluate the influence of patient weight on the cost of RA treatment with biologic agents indicated as first-line therapy after an inadequate response to MTX.
Patients and MethodsDesignA model for calculating the acquisition and administration costs was designed using Microsoft® Excel®. The DMARDs included were SC ABA, IV ABA, ADA, CZP, ETN, GLM, IFX and TCZ. Anakinra was excluded because of its limited use in clinical practice. We established a 2-year time horizon for which the induction doses and possible dose escalation were always considered in the first year.
To determine the demographic characteristics of patients with RA in Spain, retrospective data on gender, age and body weight were collected on a consecutive series of 66 patients treated in a 1000-bed tertiary care hospital in the Valencian Community in eastern Spain.
Calculation of CostsWe estimated the overall cost of DMARD therapy for an average patient whose weight coincided with the mean weight for the selected sample, as well as the difference in cost over the least costly agent, considering scenarios without and with vial optimization, that is, discarding or using what remains in partially used vials, respectively.
The costs for weight ranges of 5kg were estimated, including the minimum and maximum weights of the patients in the sample. We also calculated the cost per biologic DMARD in a hypothetical 100-patient cohort that would reflect the distribution of weights observed in the sample, assuming a normal distribution, with sample mean±standard deviation.
Resources and CostsWe took the hospital perspective, considering direct costs of acquisition of each drug and those associated with its administration. No other costs were included as we assumed that they were similar for all the alternatives. The costs of acquisition were calculated according to the doses being administered in the hospital, based on information provided by rheumatologists (Table 1), taking the cost of acquisition from the pharmaceutical company,11 applying the deduction stipulated in Spanish Royal Decree-Law8/201012 (7.5%) and adding the value added tax (4%).
Treatment Regimens Included in the Study.
| Alternative | 1st year | 2nd year |
|---|---|---|
| IV ABA | Induction phase in weeks 0, 2 and 4 of the 1st year 2 vials in patients weighing <60kg; 3 vials in those weighing ≥60kg and ≤100kg; 4 vials in those weighing >100kg 10mg/kg in a scenario of vial optimization Administration of a maintenance dose every 4 weeks A total of 14 injections | 10mg/kg in a scenario of vial optimization 2 vials in patients weighing <60kg; 3 vials in patients weighing ≥60kg and ≤100kg; 4 vials in those weighing >100kg Administration of a maintenance dose every 4 weeks A total of 13 injections |
| SC ABA | Administered in 52 weekly doses of 125mg No intravenous loading dose | Administered in 52 weekly doses of 125mg |
| ADA | 26 doses of 40mg administered at 2-week intervals | 26 doses of 40mg administered at 2-week intervals |
| CZP | Initial doses of 400mg in weeks 0, 2 and 4 Thereafter, 200mg are administered every 2 weeks Administration of 3 doses of 400mg and 23 of 200mg | Administered in 26 doses of 200mg |
| ETN | Administration of 52 doses of 50mg | Administration of 52 doses of 50mg |
| GLM | Administration of 12 monthly doses of 50mg | Administration of 12 monthly doses of 50mg |
| IFX | 3 induction doses of 3mg/kg during the first 6 weeks After week 6, doses of 3mg/kg every 8 weeks, administration of 5 doses | Administration of 7 doses of 4mg/kg |
| TCZ | Administration of doses of 8mg/kg every 4 weeks, for a total of 13 doses | Administration of doses of 8mg/kg every 4 weeks, for a total of 13 doses |
IV ABA: intravenous abatacept; SC ABA: subcutaneous abatacept; ADA: adalimumab; CZP: certolizumab pegol; ETN: etanercept; GLM: golimumab; IFX: infliximab; TCZ: tocilizumab.
The cost of IV DMARD administration was calculated on the basis of the technical specifications (IV ABA, 30min; TCZ, 60min; IFX, 60–120min).13–15 The unit costs associated with administration were obtained from a Spanish health costs database.16 The costs were expressed in 2013 euros, without taking into consideration an annual discount rate (Table 2).
Unit Costs Employed in the Cost Estimation.
| Unit pharmacy costs | ||||
|---|---|---|---|---|
| Pharmaceutical company | Administration route | Presentation | AAC per package, € | |
| Active ingredient Brand name | ||||
| Abatacept Orencia® | [1,0]BMS | IV | 250mg/vial; 1 vial per package | 334.82 |
| SC | 125mg/vial; 1 vial per package | 840.72 | ||
| Certolizumab pegol Cimzia® | UCB Pharma | SC | 200mg/syringe; 2 syringes per package | 948.00 |
| Golimumab Simponi® | MSD | SC | 50mg/pen; 1 pen per package | 1117.00 |
| Etanercept Enbrel® | Pfizer | SC | 50mg/vial; 4 vials per package | 947.22 |
| Infliximab Remicade® | MSD | IV | 100mg/vial; 1 vial per package | 536.28 |
| Tocilizumab RoActemra® | Roche | IV | 80mg/vial; 1 vial per package | 139.60 |
| 200mg/vial; 1 vial per package | 349.00 | |||
| Costs of administration of intravenous drugs | ||
|---|---|---|
| Active ingredient | Personnel costs | Unit cost, € |
| Abatacept | Drug infusion less than 1/2h | 123.76 |
| Infliximab | Drug infusion between 1/2h and 2h | 151.25 |
| Tocilizumab | Drug infusion between 1/2h and 2h | |
AAC, average acquisition cost; BMS, Bristol-Myers Squibb; IV, intravenous; MSD, Merck Sharp & Dohme; SC, subcutaneous.
Two sensitivity analyses were performed: (1) utilization of doses of 3mg/kg in the 2nd year in patients treated with IFX, instead of the 4mg/kg of the base case; and (2) utilization of doses of 6mg/kg in the 2nd year in patients treated with TCZ, rather than the 8mg/kg of the base case.
ResultsWe analyzed the data of a consecutive series of 66 patients (24% men), with a mean age of 56.8±11.7 years and mean weight of 69.0±13.1kg (range 44–103kg); 30% of the patients weighed between 65 and 74kg and 77% weighed between 55 and 84kg.
Cost Analysis Corresponding to the Average PatientIn the case of an average patient, in the scenario without vial optimization, SC ABA was the least costly agent (€21,028.09) over 2 years (Table 3). With vial optimization, the least costly drug was IFX (€20,779.29). Subcutaneous ABA was the least costly during the 2nd year (€10,514.04) (Table 4).
Costs per Patient Weighing 69kg Without Vial Optimization, €.
| IV ABAb | SC ABA | ADA | CZP | GLM | ETN | IFXb | TCZb | |
|---|---|---|---|---|---|---|---|---|
| Costs of 1st and 2nd years of treatment | ||||||||
| Total | 29,431.36 | 21,028.09 | 25,719.59 | 25,535.33 | 25,789.30 | 23,691.87 | 25,484.31 | 28,374.23 |
| Difference with respect to SC ABAa | 8403.28 | 4691.50 | 4507.24 | 4761.21 | 2663.78 | 4456.22 | 7346.14 | |
| Difference with respect to SC ABA, %a | 40.0% | 22.3% | 21.4% | 22.6% | 12.7% | 21.2% | 34.9% | |
| Second year (maintenance) | ||||||||
| Total | 14,170.66 | 10,514.04 | 12,859.79 | 11,855.69 | 12,894.65 | 11,845.93 | 11,892.68 | 14,187.11 |
| Difference with respect to SC ABAa | 4746.66 | 2345.75 | 3165.60 | 2380.60 | 1331.89 | 3077.59 | 3673.07 | |
| Difference with respect to SC ABA, %a | 45.1% | 22.3% | 30.1% | 22.6% | 12.7% | 29.3% | 34.9% | |
ADA, adalimumab; CZP, certolizumab pegol; ETN, etanercept; GLM: golimumab; IFX: infliximab; IV ABA, intravenous abatacept; SC ABA, subcutaneous abatacept; TCZ: tocilizumab.
Costs per Patient Weighing 69kg With Vial Optimization, €.
| IV ABAb | SC ABA | ADA | CZP | GLM | ETN | IFXb | TCZb | |
|---|---|---|---|---|---|---|---|---|
| Costs of 1st and 2nd years of treatment | ||||||||
| Total | 27,344.18 | 21,028.09 | 25,719.59 | 25,535.33 | 25,789.30 | 23,691.87 | 20,779.29 | 28,025.06 |
| Difference with respect to IFXa | 6564.89 | 248.80 | 4940.30 | 4756.04 | 5010.01 | 2912.58 | 7245.77 | |
| Difference with respect to IFX, %a | 31.6% | 1.2% | 23.8% | 22.9% | 24.1% | 14.0% | 34.9% | |
| Second year (maintenance) | ||||||||
| Total | 13,165.71 | 10,514.04 | 12,859.79 | 11,855.69 | 12,894.65 | 11,845.93 | 11,025.96 | 14,012.53 |
| Difference with respect to SC ABAa | 2651.67 | 2345.75 | 1341.64 | 2380.60 | 1331.89 | 511.92 | 3498.49 | |
| Difference with respect to SC ABA, %a | 25.2% | 22.3% | 12.8% | 22.6% | 12.7% | 4.9% | 33.3% | |
ADA, adalimumab; CZP, certolizumab pegol; ETN, etanercept; GLM: golimumab; IFX: infliximab; IV ABA, intravenous abatacept; SC ABA, subcutaneous abatacept; TCZ: tocilizumab.
We calculated the total 2-year cost per patient for each of the alternatives according to weight. Without optimization, IFX was the least costly in patients weighing 44–49kg, IV ABA in patients weighing 50–59kg and SC ABA from 60kg on. With optimization, IFX was the least costly in patients weighing 69kg or less and SC ABA in those weighing ≥70kg.
These results were slightly sensitive to the maintenance of the IFX dose of 3mg/kg throughout the 2nd year. Without optimization, IFX was the least costly drug in patients weighing less than 64kg, whereas, with optimization, IFX was the least costly at weights under 84kg. The reduction of the TCZ dose to 6mg/kg did not change the results.
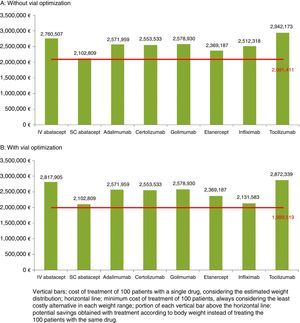
Estimate of the Savings Resulting From Treating According to Patient WeightAssuming a normal distribution of body weight, in a hypothetical sample of 100 patients, we estimated that the majority would weigh 60kg or more. Fig. 1 shows the cost of treatment of that cohort according to weight distribution both without (1A) and with (1B) vial optimization.
Both without optimization (€21,398 [€2,102,809−€2,081,411] in 100 patients) and with optimization (€113,290 [€2,102,809−€1,989,519] in 100 patients) the smallest savings corresponded to SC ABA, which was the alternative with the lowest cost and that which came nearest to the minimum cost. Without optimization, the maximum possible savings were over €21,000 per patient weighing 100–104kg treated with SC ABA instead of TCZ (Table 5). With optimization, the maximum savings achieved were over €19,000 per patient weighing 100–104kg treated with SC ABA instead of TCZ (Table 6).
Estimated Savings per Patient Over 2 Years of Treatment With the Least Costly Alternative Based on the Cost per Patient Without Vial Optimization, €.
| Weight (kg) | IV ABA | SC ABA | ADA | CZP | GLM | ETAN | IFX | TCZ |
|---|---|---|---|---|---|---|---|---|
| 45–49 | 2988.96 | 3282.30 | 7973.80 | 7789.54 | 8043.51 | 5946.08 | NA | 3645.09 |
| 50–54 | NA | 293.34 | 4984.84 | 4800.58 | 5054.55 | 2957.12 | 622.35 | 4147.80 |
| 55–59 | NA | 293.34 | 4984.84 | 4800.58 | 5054.55 | 2957.12 | 622.35 | 4147.80 |
| 60–64 | 8403.28 | NA | 4691.50 | 4507.24 | 4761.21 | 2663.78 | 329.01 | 7346.14 |
| 65–69 | 8403.28 | NA | 4691.50 | 4507.24 | 4761.21 | 2663.78 | 4456.22 | 7346.14 |
| 70–74 | 8403.28 | NA | 4691.50 | 4507.24 | 4761.21 | 2663.78 | 4456.22 | 9091.98 |
| 75–79 | 8403.28 | NA | 4691.50 | 4507.24 | 4761.21 | 2663.78 | 8067.53 | 10,837.81 |
| 80–84 | 8403.28 | NA | 4691.50 | 4507.24 | 4761.21 | 2663.78 | 8067.53 | 10,837.81 |
| 85–89 | 8403.28 | NA | 4691.50 | 4507.24 | 4761.21 | 2663.78 | 8067.53 | 14,329.49 |
| 90–94 | 8403.28 | NA | 4691.50 | 4507.24 | 4761.21 | 2663.78 | 8067.53 | 16,075.33 |
| 95–99 | 8403.28 | NA | 4691.50 | 4507.24 | 4761.21 | 2663.78 | 8067.53 | 17,821.16 |
| 100–104 | 17,099.89 | NA | 4691.50 | 4507.24 | 4761.21 | 2663.78 | 15,806.05 | 21,312.84 |
ADA, adalimumab; CZP, certolizumab pegol; ETN, etanercept; GLM: golimumab; IFX: infliximab; IV ABA, intravenous abatacept; NA, not applicable; SC ABA, subcutaneous abatacept; TCZ: tocilizumab.
Estimated Savings per Patient Over 2 Years of Treatment With the Least Costly Alternative Based on the Cost per Patient With Vial Optimization, €.
| Weight (kg) | IV ABA | SC ABA | ADA | CZP | GLM | ETAN | IFX | TCZ |
|---|---|---|---|---|---|---|---|---|
| 45–49 | 4972.97 | 5614.17 | 10,305.67 | 10,121.41 | 10,375.38 | 8277.95 | NA | 5627.79 |
| 50–54 | 5370.95 | 4272.83 | 8964.33 | 8780.07 | 9034.04 | 6936.61 | NA | 6032.29 |
| 55–59 | 5768.93 | 2931.48 | 7622.99 | 7438.72 | 7692.69 | 5595.26 | NA | 6436.78 |
| 60–64 | 6166.91 | 1590.14 | 6281.64 | 6097.38 | 6351.35 | 4253.92 | NA | 6841.27 |
| 65–69 | 6564.89 | 248.80 | 4940.30 | 4756.04 | 5010.01 | 2912.58 | NA | 7245.77 |
| 70–74 | 8055.41 | NA | 4691.50 | 4507.24 | 4761.21 | 2663.78 | 1092.55 | 8742.81 |
| 75–79 | 9794.73 | NA | 4691.50 | 4507.24 | 4761.21 | 2663.78 | 2433.89 | 10,488.65 |
| 80–84 | 11,534.06 | NA | 4691.50 | 4507.24 | 4761.21 | 2663.78 | 2702.16 | 10,837.81 |
| 85–89 | 13,273.38 | NA | 4691.50 | 4507.24 | 4761.21 | 2663.78 | 5116.58 | 13,980.32 |
| 90–94 | 15,012.70 | NA | 4691.50 | 4507.24 | 4761.21 | 2663.78 | 6457.92 | 15,726.16 |
| 95–99 | 16,752.03 | NA | 4691.50 | 4507.24 | 4761.21 | 2663.78 | 7799.26 | 17,472.00 |
| 100–104 | 18,491.35 | NA | 4691.50 | 4507.24 | 4761.21 | 2663.78 | 9140.61 | 19,217.83 |
ADA, adalimumab; CZP, certolizumab pegol; ETN, etanercept; GLM: golimumab; IFX: infliximab; IV ABA, intravenous abatacept; NA, not applicable; SC ABA, subcutaneous abatacept; TCZ: tocilizumab.
The study methodology corresponds to a cost estimate of different alternatives without analyzing efficacy, assuming that the efficacies of the treatment alternatives compared in the study have been demonstrated to be high and similar.17–22 The analysis included all the biologic DMARDs employed as first-line therapy after an inadequate response to MTX, excluding anakinra because of its limited use in routine clinical practice.
In the estimate of the overall 2-year cost per average patient, SC ABA proved to be the least costly biologic agent in the scenario without IV vial optimization (€21,028), whereas the least costly drug in the alternative scenario was IFX (€20,779). In both scenarios, SC ABA was less costly than IFX during the second year of treatment, due to the escalation of the dose of IFX from 3mg/kg to 4mg/kg. This escalation occurs frequently in clinical practice,23,24 suggesting that with a time horizon longer than that considered here, SC ABA would be the least costly alternative. This finding is consistent with a recent study that analyzed the cost of therapy and showed SC ABA to be less costly than IV ABA, ADA, CZP, ETN, GLM, IFX and TCZ in RA patients initiating treatment with biologic DMARDs.25
The analysis by weight range indicated that patient weight is a determinant of cost, as the consideration of the least costly agent varies according to this factor and, thus, does not coincide with the initial case of 69kg. The consideration of patient weight can result in savings in patients weighing 60–70kg treated with SC ABA of €2600 to €9000, depending on the alternative chosen.
In the analysis, we decided to evaluate 2 scenarios according to the use made of open IV vials as optimization is becoming increasingly widespread.26 This is only applicable in the treatment of very prevalent diseases and/or in centers that can schedule a critical number of patients to be treated simultaneously. It is an optimal situation that should be seen as a conservative premise since, in clinical practice, it does not seem to be feasible to completely avoid wastage by arranging for an exact number of patients to coincide for treatment.
Among the limitations of this study, we should point out the representativeness of the weight distribution employed in the RA patient population. In the analysis of the sample of 66 available patients, we assumed a normal distribution of the weight defined by the mean±standard deviation of the sample. This may not be an exact reflection of the RA population in Spain. Likewise, in this sample, we did not consider variables that might be relevant to body weight, such as the loss of muscle mass experienced by patients with RA as their disease progresses. Nevertheless, we considered that, with the sample analyzed, it would be possible to achieve the objective of the study, which was to examine the influence of patient weight on the cost of treatment.
On the other hand, the analysis carried out assumed that SC drugs would not have to use health care resources for their injection, although it is probable that certain patients (those of advanced age or having fear of needles) present to a health center to request the administration of the drug. The authors consider that the percentage of patients of this type would be low and that the results would not be modified in a relevant manner.
Another limitation is the consideration that all other costs would be the same among the alternatives. The AMPLE trial8 demonstrated that SC ABA is associated with a lower percentage of reactions at the injection site than ADA, a fact that would result in an unplanned difference in costs. On the other hand, as discounting was not applied, the costs of the second year with respect to the first may be underestimated, a circumstance that would favor agents with a higher maintenance cost.
Likewise, the costs of MTX and folic acid are not included as it is assumed that they are used with all the biologic DMARDs, as is suggested by the latest European clinical guidelines.6 Finally, it should be pointed out that we have not included monitoring costs, which may differ from one drug to another; the reason for excluding these costs lies in the fact that differences in monitoring costs are marginal in comparison with the costs of drug acquisition or administration.
Another possible limitation is the marked variability in the use of drugs in RA. In fact, up to 45.7% of the patients may ultimately have their doses reduced,27 and the relevance of this circumstance was pointed out in a 2014 consensus document.28 The attempt has been made to mitigate this limitation with the sensitivity analysis, which confirmed the relevance of dosage in the cost demonstrated in studies on strategies to reduce the dose or increase the intervals between treatments.23,29 This analysis involved only the IV drugs, as the study focuses on changes in costs related to body weight.
This report shows that patient weight may a factor to be considered in the prescription of biologic DMARDs and may enable a rational and more efficient allocation of resources. In view of the results obtained, to achieve a more rational use based on patient weight, the choice would be to administer IFX to patients weighing 45–49kg, IV ABA to patients weighing 50–60kg and SC ABA to patients weighing ≥60kg without IV vial optimization. With optimization, the options would be IFX in patients <70kg and SC ABA in patients ≥70kg.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of Interest/FundingThis work was funded by Bristol-Myers Squibb (BMS). Cristina Canal was employed by BMS at the time of study. Itziar Oyagüez and Manuel Gómez Barrera are employees of PORIB, a consultant specialized in the area of economic evaluation of health technology, who received funding from BMS for this article. José Andrés Román Ivorra, head of the rheumatology department, and Emilio Monte-Boquet, pharmacist, at Hospital Universitario and Politécnico La Fe de Valencia, Spain, have received fees from BMS for their advice on the development of this project. José Ivorra, rheumatologist at Hospital Universitario y Politécnico La Fe de Valencia, declares that he has no conflicts of interest. The financial support for this project has not interfered in its development.
The authors thank the reviewers of Reumatología Clínica for the comments they conveyed to us during the reviewing of the manuscript.
Please cite this article as: Román Ivorra JA, Ivorra J, Monte-Boquet E, Canal C, Oyagüez I, Gómez-Barrera M. Análisis de costes de la utilización de fármacos biológicos para la artritis reumatoide en primera línea de tratamiento tras respuesta inadecuada a metotrexato en función del peso de los pacientes. Reumatol Clin. 2016;12:123–129.