To develop and assess the feasibility in daily practice of four comorbidity checklists, for common use in rheumatoid arthritis (RA), axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA).
MethodsA multidisciplinary panel of experts on comorbidity was established. Data from the GECOAR, GECOAX and GECOAP projects were analysed and a narrative literature review in Medline on RA, axSpA and PsA comorbidity was performed in order to select the most relevant and common comorbidities across the three diseases. With these results and those obtained from a focus group of patients, in a nominal group meeting, the experts generated preliminary checklists. These were afterwards modified by an external evaluation by two associations, a patients’ association and an association of health professionals related to rheumatology. As a result, the final checklists were generated. A cross-sectional study was conducted to test the feasibility of three of the checklists in daily practice, in which eight health professionals evaluated the checklists in five patients with RA, five with axSpA and five with SpA.
ResultsFour comorbidity checklists were designed, three for health professionals (one to assess current comorbidity, one on prevention/health promotion and one with the referral criteria to other health professionals), and another for patients. The feasibility study showed them to be simple, clear, and useful for use in routine clinical practice.
ConclusionsThe use of specific and common checklists for patients with RA, axSpA and PsA is feasible and might contribute favorably to their prognosis as well as in daily practice.
Desarrollar y analizar la viabilidad en la práctica diaria de cuatro checklists relacionados con la comorbilidad, comunes para pacientes con artritis reumatoide (AR), espondiloartritis axial (EspAax) y artritis psoriásica (APs).
MétodosSe estableció un grupo multidisciplinar de expertos en comorbilidad. Se revisaron los proyectos GECOAR, GECOAX y GECOAP, y se realizó una búsqueda bibliográfica en Medline sobre comorbilidad en AR, EspAax y APs, para seleccionar las comorbilidades más relevantes y comunes a las tres enfermedades. Con estos resultados y los obtenidos de un grupo focal de pacientes, en una reunión de grupo nominal, los expertos generaron unos checklists preliminares. Estos listados preliminares se modificaron, tras una evaluación externa por una asociación de pacientes y otra de profesionales de la salud relacionados con la reumatología, para generar los checklists definitivos. Finalmente, se realizó un estudio transversal, en el que ocho profesionales de la salud evaluaron tres checklists en cinco pacientes con AR, cinco con EspAax y cinco con APs.
ResultadosSe diseñaron cuatro checklists de comorbilidad, tres para profesionales de la salud (uno sobre evaluación de la comorbilidad presente, otro sobre prevención/promoción de la salud y un último con los criterios de derivación a otros profesionales), y otro para pacientes. El estudio de viabilidad mostró que son sencillos, claros y útiles para su uso en la práctica clínica habitual.
ConclusionesEl uso de checklists específicos y comunes para pacientes con AR, EspAax y APs es factible y puede contribuir favorablemente en su pronóstico así como en la práctica clínica habitual.
Rheumatoid arthritis (AR), axial spondyloarthritis(AxSpA) and psoriatic arthritis (PsA) are common chronic inflammatory diseases in the general population.1–3
Furthermore, this group of diseases has been shown to have a high prevalence of comorbidities,4 many of them common to the three entities, where comorbidity and cardiovascular (CV) risk factors are notable. Thus, for example, the COMORA study, which was a transversal study conducted in 17 countries with almost 4000 patients with RA, demonstrated high blood pressure (HBP) prevalence of 40.4%, hypercholesterolaemia of 31.7% and 15% depression in these patients.5 The COMOSPA study, which was similar to the previous one, was conducted in 22 countries and assessed over 3000 consecutive patients with axial spondyloarthritis and reported the prevalence of different comorbidities.6 A prevalence of 33.5% HBP was estimated, together with 29.3% for a tobacco habit, 27.3% for hypercholesterolaemia, 13% osteoporosis, 11% gastroduodenal ulcer and 4% for CV events. Similar findings were reported in the Spanish population.7 Finally, in patients with PsA prevalence of HBP, Diabetes Mellitus (DM), obesity and metabolic syndrome have been reported up to 37%, 12%, 30% and 40%, respectively.8,9
Also, different publications have shown the great impact comorbidity has had on these patients.10,11 In one prospective cohort of patients with RA it was demonstrated that the higher the number of comorbidities, the pootrt the functional status, regardless of disease activity.12 The CARMA study also reported in patients with ankylosing spondylitis and PsA an independent association between comorbidity and a poorer functional status.13
Due to all of the above, different projects have been developed to address the management of comorbidity in these illnesses. These include the GECOAR Project in RA,14 GECOAX in AxSpA15 and GECOAP in PsA,16 and aim to promote a framework in management. They all contain explicit recommendations and even other materials for the identification, assessment and control of the comorbidity. The fact that there are specific approaches may be highly beneficial in clinical practice since they are very much aimed at each type of patient. However, on the other hand, the lack of time, and especially in those centres with a high healthcare load, this may be a limiting factor when following the recommendations of these documents.
Based on the above, and bearing in mind that the three diseases share many comorbidities, the aim of the GECOAI Project (inflammatory arthritis comorbidity management) was to create common support documents for the three diseases for all health professionals involved in the management of these conditions, and also for the patients. The purpose was to more effectively manage possible associated comorbidities and therefore reduce the variability in the management of patients with RA, AxSpA and PsA.
MethodsStudy designThis was a mixed design study (qualitative and quantitative). For the development of the checklists qualitative study methodology was followed based on narrative review of the literature, a focus group of patients and a nominal group of experts. For the feasibility study a cross-sectional observation study was conducted. The project was carried out in keeping with the good clinical practices regulations/guidelines and the current version of the revised declaration of Helsinki. It also had the endorsement of the Rheumatology Society of the Community of Madrid (SORCOM), OPENREUMA and the National Arthritis Coordinators (ConArtritis).
Participant selectionIn the first place a multidisciplinary group of healthcare professionals was established with interest and experience in the management of patients with RA, AxSpA and PsA, formed by seven rheumatologists (three of them were the project coordinators), two primary care physicians, an internal medicine expert in CV comorbidity, three nurses and one psychologist.
Development of checklistsDuring the first phase three main documents were reviewed14–16 and a narrative review of the literature was made in search of articles on the management of comorbidity in RA, AxSpA and PsA. Recrodings were made of the comorbidities to assess in normal practice, their form of assessment and their rates. The Pubmed Clinical Queries were used, and small strategies of search using Mesh and free text terms. All this information was presented and discussed with the three project coordinators who selected the comorbidities and elements considered relevant (in relation to prevalence and impact of comorbidity or capacity for its prevention) and cross-sectiontional (common to the three diseases). These inclucded pharmacological allergies, current medication and possible related problems (tolerance, adherence, etc.), together with CV comorbidities and CV risk factors, infections and vaccines, depression, osteoporosis, gastrointestinal comorbidities and others, such as uveitis. After this, the previously exposed results were presented in a focus group of patients with RA, PsA and AxSpA, for which a typological box was designed to include heterogeneous and representative people. The focus group lasted 95min and an audio recorder was used to record the detailed discussion. The following aspects, among others, were discussed: medical and patient terminology related to comorbidities; their opinion on which comorbidities would be important to know about, and comprehensible ways of identifying and reporting them.
Everything that was collected in the previous steps was later addressed in a nominal group meeting in which the multidisciplinary health professionals took part, with the exception of the three coordinators. After reviewing all data consensus was reached to create three types of checklists, a specific one for patients, aimed at facilitating posterior assessment by health professionals, and another three specific ones for the healthcare professionals. One was for assessment of current comorbidity, another focused on comorbidity prevention and health promotion and the last on assessment for possible referral to another healthcare professional. The variables to be included in each checklist and their type of evaluation were also agreed. These were then produced and set up in a preliminary fashion for use in daily practice.
The specific comorbidity checklist for patients was analysed by ConArtritis (its objectives, content and clarity) and all the checklists by OPENREUMA. Both associations evaluated the aims, their content and their clarity. The comments of the two associations were reviewed and considered by the coordinators who defined the final checklists which were formatted in their final version.
Feasibility studyFeasibility in clinical practice of the three checklists relating to healthcare professionals was confirmed by a small cross-sectional study. Eight members of the multi disciplinary group (five rheumatologists and three nurses) took part. Each of them applied the three checklists to 15 patients (five with RA, five with AxSpA, and five with PsA) from their regular practice. After this, they filled in a specific data collection book which included: 1) Centre characteristics; service (including members and presence of monographic consultations of RA, AxSpA and PsA); characteristics of the professional; 2) checklist characteristics relating to time of completion (in minutes); simplicity, user-friendliness and general usefulness, recorded on a scale of 0 (not at all) to 10 (very, a lot); impact in daily practice improvement (assessing of comorbidity, prevention of the same and referral to other healthcare professionals) and its need for review were responded to with yes/no; 3) Other factors, such as its recommendation to other colleagues (yes/no) and further comments.
Statistical analysisFor the feasibility study a descriptive study was made on the data. The quantitative variables were described using mean and standard deviation and the qualitative with frequencies and percentages.
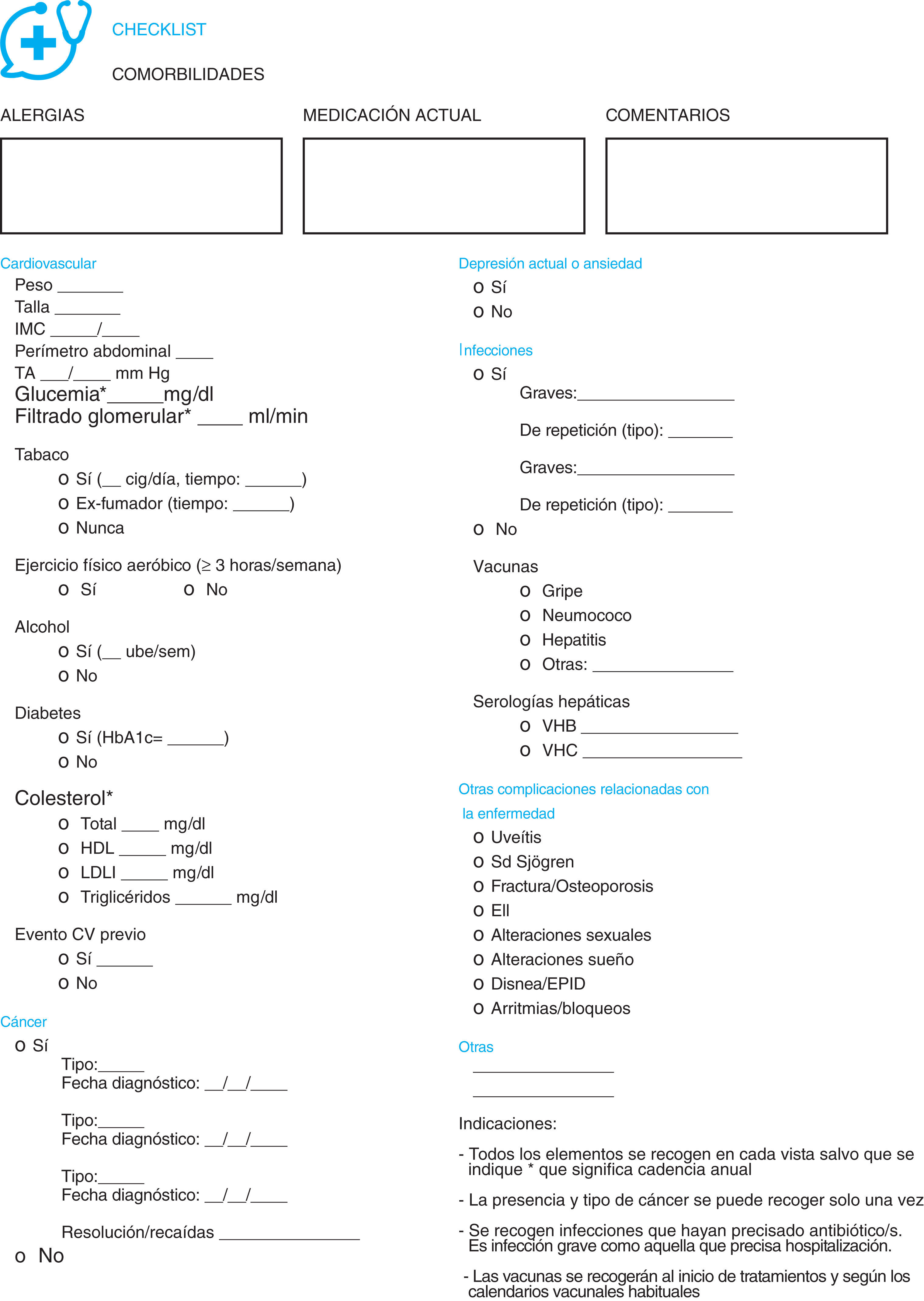
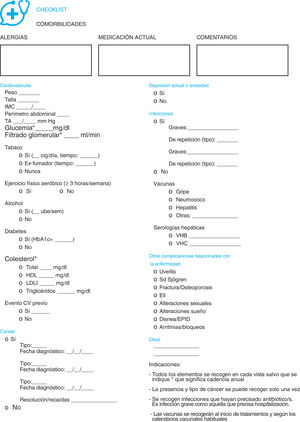
ResultsChecklistsFour checklists were created relating to comorbidity, one specifically for patients (Fig. 1) and three for health professionals responsible for the care of patients with RA, AxSpA and PsA. One related to the assessment of comorbidity (Fig. 2), another to the prevention of it and health promotion (Fig. 3) and another for referral to other healthcare professionals (Fig. 4).
The checklist for patients (Fig. 1), begins with an explanation of the objectives pursued and contains 13 comprehensively formulated questions approved by the patients themselves. Relevant comorbidities are included about which both professionals and patients agreed were comprehensible and simple to reliably report on. They address the disease suffered from and the medication related to it, toxics habits (tobacco and alcohol) and, through the use of direct, and indirect questions, or scales, they assess the mood, infections, vaccines, ocular pathology and bone metabolism. They also allude to visits to emergency services since these could indicate a serious epode related to the comorbidity under study, or of some other type.
The assessment checklist of comorbidity for healthcare professionals (Fig. 2) includes, initially, three spaces for free text on pharmacological allergies, current medication and free comments (if they are considered necessary). This is followed by a section on cardiovascular pathology and CV risks factors. In this part previous events may be recorded and variables such as weight, toxic habits, concentrations of cholesterol or regular exercise. They also evaluate a history of neoplasia (type, date and status), the presence of depression, infections (including their severity, vaccines and serologies) and other complications related with AR, AxSpA and PsA. A series of general indications are provided at the end and indication on the main comorbidity assessment rate.
The comorbidity prevention and health promotion checklist (Fig. 3) includes preventable comorbidities (and how to assess/measure them), for the purpose of confirming that all the right procedures have been taken relating to the prevention of the comorbidity and the promotion of health in patients with this group of diseases. Among other factors are diet, the risk of fracture, oral hygiene, adherence to treatment and sleep and social life assessment. With regards to the format of assessment, direct questions are included as is the use of specific questionnaires such the BASDAI,17 HADS18 or the Pittsburgh Sleep Quality Index.19
Finally, the referral checklist (Fig. 4) describes the criteria which should be considered for referring a patient who has certain comorbidities to other healthcare professionals, such as nurses, other medical specialists and other professionals such as occupational therapists or psychologists.
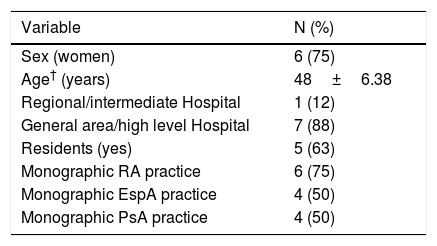
Feasibility studyA total of eight healthcare professionals from seven different centres participated in this study, in which the checklists were used on 120 patients. Table 1 shows the main characteristics of the professionals and the participant centres. Seventy five per cent were women, with a mean age of 48±6.4 years, from high care level hospitals, with a number of rheumatologists per service which varied from 3 to 12 and a mean waiting time of 20 days to two months. Seventy five per cent of these centres had a monographic RA practice and half (four centres) a practice for AxSpA and PsA.
Characteristics of the participants of the feasibility study.*.
| Variable | N (%) |
|---|---|
| Sex (women) | 6 (75) |
| Age† (years) | 48±6.38 |
| Regional/intermediate Hospital | 1 (12) |
| General area/high level Hospital | 7 (88) |
| Residents (yes) | 5 (63) |
| Monographic RA practice | 6 (75) |
| Monographic EspA practice | 4 (50) |
| Monographic PsA practice | 4 (50) |
EspA: axial spondyloarthritis; PsA: psoriatic arthritis; RA: rheumatoid arthritis.
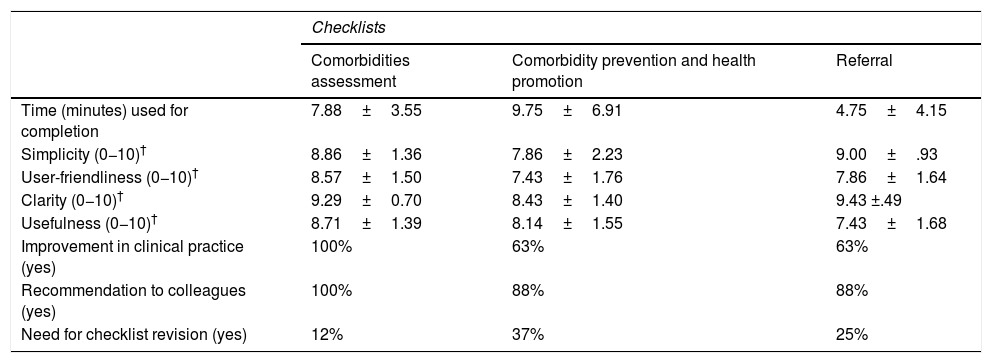
Regarding the feasibility study results (Table 2), a mean time of completion was estimated at 7.88±3.55min, 9.75±6.91min and 4.75±4.15min for the comorbidity assessment checklists, comorbidity prevention and health promotion and referral, respectively.
Results from the feasibility study of healthcare professionals checklists.*
| Checklists | |||
|---|---|---|---|
| Comorbidities assessment | Comorbidity prevention and health promotion | Referral | |
| Time (minutes) used for completion | 7.88±3.55 | 9.75±6.91 | 4.75±4.15 |
| Simplicity (0−10)† | 8.86±1.36 | 7.86±2.23 | 9.00±.93 |
| User-friendliness (0−10)† | 8.57±1.50 | 7.43±1.76 | 7.86±1.64 |
| Clarity (0−10)† | 9.29±0.70 | 8.43±1.40 | 9.43 ±.49 |
| Usefulness (0−10)† | 8.71±1.39 | 8.14±1.55 | 7.43±1.68 |
| Improvement in clinical practice (yes) | 100% | 63% | 63% |
| Recommendation to colleagues (yes) | 100% | 88% | 88% |
| Need for checklist revision (yes) | 12% | 37% | 25% |
Evaluation scales 0−10: 0: nothing; 10: very, a lot.
The simplicity of the three checklists was high (Table 2), with means scores of 0 (nothing) to 10 (very/a lot) of 8.86±1.36 (assessment of comorbidity), 7.86±2.23 (comorbidity prevention and health promotion) and 9.00±.93 (referral), as well as scores on user friendliness, clarity and usefulness (Table 2).
Furthermore, 100% of participants considered that the checklist of assessment of comorbidity would improve the daily practice and they would recommend it to their colleagues (Table 2). Equally, 63% said that the checklists on comorbidity prevention and health promotion and referral would have a positive impact on daily practice (to determine new comorbidities, having a more overall view of the same) and 88% would recommend it to their colleagues.
DiscussionThe high prevalence and impact of comorbidity in RA, AxSpA and PsA is widely contrasted.1,10,12,13,20
In fact, different consensuses and clinical practice guidelines’, both national and international, recommend the systematic assessment and management of it in patients with this group of diseases.21–25 However, published data indicates that this assessment is sub-optimum.26,27
As a result, in recent years many projects have been developed to revert this situation. Specifically, the projects GECOAR,14 GECOAX15 and GECOPsA,16 which were developed in Spain, and which generated a specific framework for the correct attention to comorbidity, for RA, AxSpA and PsA, in order to improve the previously described situation. Thus, data began to be published which suggested that all of these initiatives would have a positive impact on daily practice, although we still had to continue improving to achieve appropriate comorbidity assessment and management.28
There is no doubt that the current high pressured healthcare of many centres in Spain may be clearly limited to the assessment of comorbidity following the framework described for each disease.14–16 It is for this reason that the aim of the GECOAI project was to provide continuity to the approach of comorbidity and generate practical materials in the form of simple checklists of common use for this group of diseases.
For this proposal, we used what had been created from previous projects and also had help from published evidence, patient opinion and the knowledge and experience of a broad multidisciplinary group of experts. The checklists were also then assessed by a patient association and an association of healthcare professionals in rheumatology and the viability of three of them was analysed, reinforcing the validity of their contents.
We highlight as a new contribution to this project, the generation of a specific checklist for patients. This, as we have said, is based on their opinions. For its design we took into account their level of comprehension of medical language, relating to comorbidities, and their knowledge of them. Based on these factors, variables were arranged to be included in the checklist, to obtain the greatest quantity of information possible in a reliable format. An explanatory text suggested and argued by them was also included. The experts were convinced that this checklist will be of enormous help in subsequent assessment of comorbidity by healthcare professionals who attend patients.
We also wished to assess the comorbidity prevention and health promotion checklist. This was was also highly novel, and we took into account its high impact for the patient10–13 and the healthcare system,29–31 critical to daily practice. For this reason the experts wish to reinforce this point with the highest development of similar initiatives, to help prevent maximum development of comorbidities.
In relation to the assessment checklists of comorbidity and referral, we would highlight their cross-sectional value, i.e. they are checklists which may be used indistinctly in the three before-mentioned diseases. Specifically, the assessment one contains the most relevant comorbidities (according to their prevalence and impact), although space is also available to record other different ones. Also, depending on the variables, other descriptive data are collected which may be important for daily practice, accompanied by a series of easy-to-follow instructions. We would also highlight that the referral checklist is based, among other things, on criteria ageed in a document by the Spanish Society of Rheumatology on referral in rheumatology.32
At the same time, another of the project novelties, which also provides great value to clinical practice, is the viability study of the healthcare professional checklists. The checklists were used in daily practice and assessed in relation to completion time; simplicity; clarity and usefulness. In general overall evaluation was highly positive. Completion time was not excessive and the majority of professionals considered that it would have a positive impact on their daily practice (for example for determining new comorbidities, having a global vision of them or preventing them better). Curiously, the general scores for the comorbidity assessment checklist were more positive compared with the other two. This may be due to the fact that we are still not very familiar with comorbidity prevention and health promotion. In the case of referral, this may be subject to other conditioning factors related to local environment. We have already highlighted the importance of comorbidity prevention and the promotion of health in our day to day practice, and we also consider that good coordination between professionals is essential for addressing comorbidities appropriately.
The GECOAI Project does, however, have a series of limitations. The first derives from the choice of variables included in the checklists. Notwithstanding, it is improbable that something relevant is missing, since it is taken from studies which have already assessed these aspects, and also the evaluation checklists are open to the inclusion of other variables from each consultation practice. Lack of familiarity with comorbidity prevention and health promotion could be a barrier to the initial implementation of the checklists. The main limitation, however, is that we have not yet demonstrated that their use has brought about improvements to the health of the patients. However, the experts believe it is probable that this will occur. This would be the aim of future projects. We should, however, point out several aspects on the use and limitations of this type of tool in our clinical practice (often with great healthcare pressure). Just as we have described, there is not one, but three checklists, which require time for completion and this could limit their implementation in daily practice. However, in the feasibility study, the mean time employed for use was reasonable and dropped as they were familiarized. This is precisely the aim and advantage of the checklists: that the clinician is able to naturally standardise and incorporate several control tools for optimising clinical management.
To sum up, with this project we have created four checklists for the management of comorbidity to be commonly used in patients with RA, AxSpA and PsA, and which are simple and viable regarding implementation in daily practice. We are convinced that with them we will help to improve both the health of the patients and their healthcare. However, it is important to determine their real use in a few years time through their real implementation in clinical practice.
FinancingThis Project was financed by Merck Sharp & Dohme® España and endorsed by the following associations/societies: CONARTRITIS (National coordinator of the arthritis association of patients representing persons affected by RA, PsA, JIA and EspA), OPENREUMA (Association of other healthcare professionals dedicated to rheumatology) and SORCOM (Rheumatology Society of the Community of Madrid). Merck Sharp & Dohme® had no impact on project development or the final manuscript content.
Conflict of interestsMG has collaborated as consultant or researcher with MSD®, Abbvie®, Sanofi® and Pfizer®. SC has received research grants or aids from MSD®, Pfizer® and Roche®. In recent years they have also received occasional fees for papers and/or consultations from Abbvie®, Amgen®, BMS®, Celgene®, Gebro-Pharma®, Janssen®, Lilly®, MSD®, Pfizer®, Roche®, Stada®, Theramex® and UCB®.
The authors would like to thank Dr. Ana M. Ortiz, from the rheumatology unit of the University Hospital La Princesa, Madrid, for her comments and constructive criticisms of the checklists.
Please cite this article as: Castañeda S, González C, Villaverde V, Lajas Petisco C, Castro MC, Jirout F, et al. Desarrollo y viabilidad de cuatro checklists para la evaluación de la comorbilidad en pacientes con artritis reumatoide, espondiloartritis axial y artritis psoriásica: Proyecto GECOAI. Reumatol Clin. 2022;18:114–123.