Pulmonary haemorrhage (PH) in systemic lupus erythematosus (SLE) is a rare but potentially fatal complication due to its high mortality. Early treatment benefits the outcome.
Reports on predictive factors of PH in SLE patients are scarce.
ObjectiveTo describe a case series of PH in SLE patients that were attended in the Rheumatology Section of the J. M. Cullen Hospital and to compare this data with published results.
MethodsPatients with SLE (1982–1997 ACR criteria) and PH diagnosed by clinical criteria (cough, dyspnoea, haemoptysis), haemoglobin below 12 g/dL or drop greater than 2 points, new radiological infiltrate and bronchioalveolar lavage, monitored between June 1987 and December 2019 were studied. Demographic, clinical, laboratory, treatment and prognosis data related to PH were analysed.
ResultsFrom a database of 306 SLE patients, 25 (8.2%) developed 29 episodes of PH. PH was the first manifestation of SLE in 8 patients. Renal involvement was the most frequent manifestation prior to the development of PH. SLE activity (measured by SLEDAI) was high during the episodes (mean: 16.8). Renal failure (p = .027) and mechanical respiratory support (p = .006) were related to mortality (40.7%) with statistical significance. Patients with SLEDAI higher to 10 at SLE onset showed more likelihood of developing PH. The OR was 2.68 (p = .046).
ConclusionsAlthough treatment in SLE has progressed in recent years, PH continues to be a rare and severe complication of this disease. When a PH is suspected, studies to confirm it must be done rapidly, since early diagnosis and aggressive treatment have been shown to improve survival. We observed that patients with renal involvement and mechanical respiratory support had higher mortality than SLE patients without them.
La hemorragia alveolar difusa (HAD) es una complicación infrecuente pero grave en pacientes con Lupus eritematoso sistémico (LES). Su tratamiento debe ser precoz, lo cual mejora la supervivencia. Las comunicaciones de factores predictores de HAD en pacientes con LES son escasas.
ObjetivoDescribir una serie de casos de HAD en pacientes con LES, del Servicio de Reumatología del Hospital J.M. Cullen, de Santa Fe, y compararlos con un grupo control de pacientes con LES del mismo Servicio y con los datos de la literatura.
Material y métodosSe incluyeron pacientes con LES (Criterios ACR 1982–1997) y HAD definida por parámetros clínicos (tos, disnea, hemoptisis), analíticos (caída de la hemoglobina por debajo de 12 g/dL o mayor a dos puntos respecto del basal en pacientes ya conocidos), imagenológicos (infiltrado radiológico y/o tomográfico bilateral o difuso) y lavado bronquioalveolar (BAL) (retorno sanguinolento en el lavado, más de 20% de siderófagos, sin evidencia de lesiones sangrantes), quienes concurrieron al servicio entre junio de 1987 y diciembre de 2019. Se analizaron datos demográficos, clínicos, de laboratorio, tratamientos y pronóstico de los pacientes.
ResultadosSe trabajó con una base de datos de 306 pacientes con diagnóstico de LES, evaluándose 25 de ellos (8,2%) que presentaron 29 episodios de HAD (ocho de ellos como forma de inicio de la enfermedad). El compromiso renal fue el más frecuentemente asociado a la HAD (previo o concomitantemente). La actividad de la enfermedad medida por SLEDAI fue alta durante el episodio, y su media fue de 16,8 puntos. En todos los casos se constató sangrado pulmonar por BAL o tubo endotraqueal. Se halló significación estadística al relacionar la mortalidad (40,7%) con requerimiento de asistencia respiratoria mecánica (ARM) (p = 0,006) y falla renal (p = 0,027). Los pacientes con SLEDAI mayor a 10 al inicio de la enfermedad presentaron más posibilidades de desarrollar HAD (OR = 2,68, p = 0,046).
Todos los pacientes recibieron metilprednisolona en pulsos y en menor porcentaje ciclofosfamida y plasmaféresis.
ConclusiónA pesar de los avances en los últimos años, en relación con el tratamiento del LES, sigue siendo alta la mortalidad de la hemorragia pulmonar. Sospechar su presencia nos obliga a estudiar rápidamente a estos pacientes, dado que el diagnóstico temprano y el tratamiento intensivo han demostrado mejorar la supervivencia. Hemos observado que aquellos pacientes con requerimiento de ARM y compromiso de la función renal son quienes presentan un mayor índice de mortalidad de manera estadísticamente significativa.
The term diffuse alveolar haemorrhage (DAH) covers a series of clinical entities which lead to secondary pulmonary haemorrhage from pulmonary microvasculature (alveolar capillaries, arterioles and venules) lesions in different areas and is often generalised.1 It groups together a wide range of pathologies which may be limited to the lung or involve other organs such as the kidney (kidney–lung syndrome). The DAH syndrome may present with dyspnoea, cough and haemoptysis, or have an onset which is more overlapping or subclinical, only evidenced by a drop in haemoglobin. As a result, a rapid and often invasive methodology is required to reach definitive diagnosis since early treatment has been proven to be the only method of improving survival.
DAHs are usually classified in relation to histological symptoms concerning capillaries (present or not) and physiopathology (such as ANCA-associated pauci-immune vasculitis, deposits of immunocomplexes and a wide and varied range of other causes such as drugs, infections, etc.). Given the low frequency of DAH, few case series exist assessing their aetiology. Some cases series, such as that of Travis et al.,2 which presented 37 patients and Buendía Roldán et al.,3 with 17 patients, observed that the most common causes of DAH were ANCA-associated vasculitis. Ortiz et al. presented 14 patients with connective tissue diseases and DAH where SLE was the baseline disease in 11.4
DAH is a complication of very low prevalence in patients with systemic lupus erythematosus (SLE). It may be observed during evolution in 2%–5% of lupus patients and as an initial manifestation in 11%–20%.3 Although its description is long in data (Osler in 1904),5 the same presented little advance in treatment compared with other SLE complications such as renal compromise, possibly due to the low prevalence presented. It develops in patients with active baseline disease, with a mortality of up to 80%. It is clinically characterized by the presence of haemoptysis, dyspnoea, infiltrated lungs, a drop in haematocrit and fever. These manifestations, associated with new infiltrates in radiography of the chest, are suggestive of diagnosis, especially in patients already diagnosed with SLE. However, the problem stems from those cases where this is the first manifestation of the disease and in whom the medical expression of symptoms is low or absent (subclinical events), with the only evidence being a drop in haemoglobin.4 Added to this, the main symptom of haemoptysis is not the most common of symptoms communicated in 35%6 to 57%7 of episodes, even in mass hemorrhages.8 For these reasons (and the imperious need to rule out infections prior to the onset of immunosuppressant therapy), BAL acquires an essential role in the early diagnosis and correct treatment of these patients.
ObjectiveThe aim of this study was to describe the clinical characteristics, results from complementary studies and data of relevance of patients diagnosed with SLE who developed DAH in the Rheumatology Unit of the Hospital J.M. Cullen, in Santa Fe; and to conduct a comparative study of these cases with a control sample of patients without DAH from the same unit, to assess factors which predict the development of this complication and the association with mortality. We carried out a comparison with the literature with the data obtained in this study.
Material and methodsA retrospective, descriptive and cross-sectional study was conducted, using analysis from medical records (n = 306) of patients with a diagnosis of SLE (ACR 1982–1997 criteria) who complied with periodical controls in the rheumatology unit of the Hospital J.M. Cullen, in the city of Santa Fe, from 1987 to 2019. Demographic, clinical and analytical data were processed during the episode, with previous organ compromise and the most relevant characteristics of the patients relating to the baseline disease.
DAH was defined by clinical, analytical and imaging criteria: A) drop in haemoglobin (Hb) of two or more points, B) Hb under 12 g/dL, C) haemoptysis, D) hypoxemia, E) respiratory failure, F) radiological infiltrates in 3⁄4 of pulmonary fields and/or G) broncoalveolar lavage (BAL) with siderophages above 20% or the presence of blood in the endotracheal tube.9
DAH for other causes were excluded (e.g. thromboembolism of the lung, uraemia, acute pulmonary oedema) and the cases which were not confirmed by BAL or endotracheal tube.
The Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) was used to assess the baseline disease activity at onset and in the DAH episode. Renal compromise was defined as the presence of proteinuria above .5 g in 24 h, active urinary sediment, or renal failure with creatinine levels below 60 mL/min/1.73 m2; respiratory insufficiency with arterial oxygen partial pressure (PO2) under 60 mmHg; thrombocytopenia under 100,000 platelets/mL and a diagnostic delay from the time of onset of the first symptoms attributable to DAH and its confirmation through BAL.
Statistical analysis was performed with the SPSS® Statistics 19 programme and results were expressed in percentage, mean and median, as appropriate. The comparison of means and proportions was performed with the Mann Whitney U test, the Chi squared test and the exact Fisher test, with a statistical significant rate of α ≤ .05. The odds ratio (OR) was calculated from among the variables researched in search of factors which predicted the possibility of developing DAH in patients with SLE; and within these episodes, those characteristics associated with higher mortality, comparing the patients with DAH (n = 25) with a sample of control patients with SLE without DAH (n = 100) paired by sex and age.
For analysis of DAH patient evolution and complications, each DAH episode was considered different.
ResultsRegarding the evaluation of the 306 medical records of patients diagnosed with SLE, 25 of them (8.2%) presented with 29 episodes of DAH (three were recurrent). 22/25 patients were women (88%), with a mean age in the episode of 33 years with a CI (28–38). DAH presented on average at 63 months of SLE onset. Eight of the 25 patients presented with it as a first symptom of the disease, representing 32% of the sample.
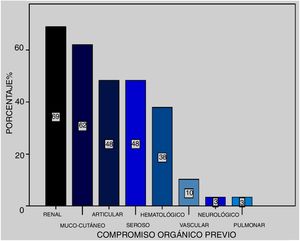
At the time of the episode, SLEDAI was 16.8 with a CI (12–21). Previous organ compromise was presented in 25 patients arranged in frequency as: renal (69%), mucocutaneous (62%), articular (48%), serous (48%), haematological (38%), vascular (10%) and neurological (3%) (Fig. 1).
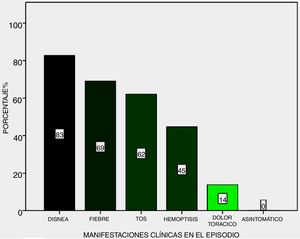
Symptoms: the most frequent during the 29 episode were dyspnoea (83%), fever (69%) and cough (62%), haemoptysis (45%) and final chest pain (14%), no haemorrhaging was subclinical (Fig. 2).
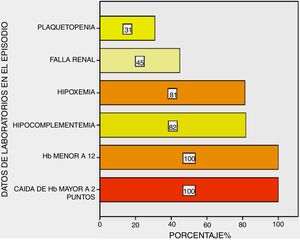
All patients presented with a drop in haemoglobin above two points and haemoglobin under 12 g/dL; hypocomplementaemia at 82%, respiratory insufficiency at 81%, changes to renal function at 45% and thrombocytopenia at 31% of them (Fig. 3).
All the patients presented with imaging compromise in the radiography and/or CAT, the most common pattern being bilateral patchy consolidations in ground-glass.
BAL was practised on all patients, except one, who was intubated on hospital admittance, with blood being observed in the endotracheal tube. The mean of siderophage findings was 35%. Six of our patients presented with positive cultures in the BAL on admittance with evidence of 3/6 Streptococcus pneumonia, 2/6 Staphylococcus aurous sensitive to meticillin and the last a Klebsiella penumoniae (with appropriate antibiotic treatment for sensitivity).
The average diagnostic delay of the DAH was 4.6 days (CI 2.5–6.7).
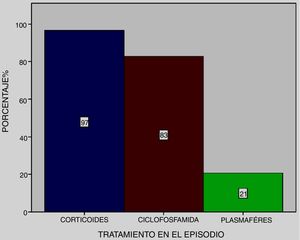
All patients received pulse methylprednisolone, together with cyclophosphamide at 83% and plasmapheresis at 21% (Fig. 4), except one who died on admittance to intensive care.
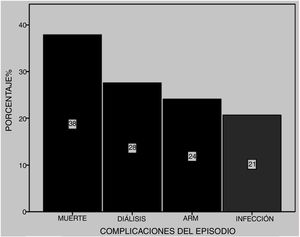
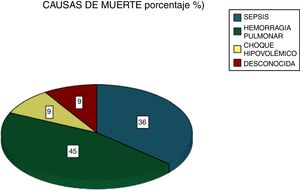
Among the most common complications were infections at 21% (five due to gram-negative bacilli and one to Candida albicans), with 28% of patients requiring dialysis and 24% MRS. Of these latter patients, seven died (Fig. 5). Six patients received plasmapheresis and four of them died. In total 11 patients died (38%), most due to pulmonary haemorrhaging (Fig. 6).
We found there was a higher statistically significant possibility of mortality in those patients whose onset of SLE was at a later age (Table 1), and in those who in the DAH episode presented with renal failure and required MRS (OR = 6.9 and p = .027 and OR = 20.4 and p = .006 respectively) (Table 2).
Main characteristics of comparison between alive and deceased patients with diffuse alveolar haemorrhage (DAH). Age at onset of disease proved to be statistically significant.
| Alive (n = 18) | Deceased (n = 11) | P | |
|---|---|---|---|
| Sex | |||
| Female | 13 | 10 | .36 |
| Male | 5 | 1 | |
| Age at onset of LES | 27.8 ± 9.8 | 31.3 ± 15.3 | .035 |
| Age at time of DAH | 31.2 ± 12.9 | 35.1 ± 12.3 | .80 |
| DAH as onset of SLE | 5/8 | 3/8 | 1.00 |
| SLEDAI in episode | 16.1 ± 9.1 | 14.9 ± 12.3 | .56 |
| Haemoptysis | 5/12 | 7/12 | .12 |
| Haematocrit (mean) | 23.7 ± 5.6 | 17.9 ± 3.7 | .45 |
| Platelets (mean) | 221,250 ± 135,953 | 162,000 ± 118,452 | .29 |
| Complement | 11/18 | 7/18 | .55 |
| Previous treatment with hydroxychloroquine | 7/9 | 2/9 | .41 |
| % of siderophages in BAL (mean) | 45.3 ± 23.3 | 37.1 ± 22.3 | .43 |
Risk factors of mortality of patients with systemic lupus erythematosus (SLE) and diffuse alveolar haemorrhage (DAH). Both renal failure and the need for mechanical respiratory support (MRS) were statistically significant, increasing the probabilities of death.
| Characteristics | Live patients | Deceased patients | p | OR |
|---|---|---|---|---|
| N = 18 | N = 11 | |||
| Kidney failure (reduction of lightening of Cr) | 5/18 (27.8%) | 8/11 (72.7%) | .027 | 6.93 (1.29−37.22) |
| MRS | 1/18 (5.6%) | 6/11 (54.5%) | .006 | 20.40 (1.96−211.79) |
| Infections | 4/18 (22.2%) | 2/11 (18.2%) | 1.00 | .78 (.12−5.16) |
A series of patients with SLE and DAH (cases) were compared with 100 patients with SLE without DAH (controls), detecting that there was haemolytic anaemia and renal compromise at the onset of the disease, being predominant in patients who presented with DAH with an OR of .22 and a p = .05 and an OR = .25 and p = .004, without increasing their possibilities and the SLEDAI above 10 points at the onset raised the opportunities of suffering from haemorrhaging 2.68 times more with p = .046. The possibility of death in patients with DAH compared with controls was 4.56 times higher with p = .005 (Table 3).
Case and control comparisons. Patients who’s SLEDAI at the onset of the disuse was higher than 10, presented with greater probabilities of developing diffuse alveolar haemorrhage (DAH) and therefore, higher mortality.
| Cases (DAH n = 25) | Controls (N = 100) | P | OR | ||
|---|---|---|---|---|---|
| Sex | Female | 3/25 (12%) | 11/100 (11%) | 1.00 | .91 (.23–3.53) |
| Male | 22/25 (88%) | 89/100 (89%) | |||
| Age at onset of SLE (years) | Over 30 years | 10/25 (40%) | 37/100 (37%) | .82 | 1.13 (.46–2.78) |
| Under 30 years | 15/25 (60%) | 63/100 (63%) | |||
| SLEDAI at onset of the disease | Under 10 | 7/25 (28%) | 51/100 (51%) | .046 | 2.68 (1.02–6.96) |
| Over 10 | 18/25 (72%) | 49/100 (49%) | |||
| Time of disease evolution | 3.8 years (DS 5.5) | 4.60 years (DS 5.4) | .82 | – | |
| Alopecia | 10/25 (40%) | 36/100 (36%) | .82 | .84 (.34–2.07) | |
| Arthritis | 13/25 (52%) | 65/100 (65%) | .25 | 1.71 (.71–4.16) | |
| Erythema malar | 17/25 (68%) | 65/100 | .82 | .87 (.34–2.23) | |
| Ulcers | 5/25 (20%) | 25/100 (25%) | .79 | 1.33 (.45–3.92) | |
| Renal | 18/25 (72%) | 39/100 (39%) | .004 | .25 (.09–.65) | |
| Serositis | 7/25 (28%) | 24/100 (24%) | .79 | .81 (.30–2.17) | |
| Neurological | 1/25 (4%) | 8/100 (8%) | .69 | 2.08 (.25–17.51) | |
| Haemolytic anaemia | 4/25 (16%) | 4/100 (4%) | .05 | .22 (.05- .95) | |
| Anti-DNA | 15/25 (60%) | 49/100 (49%) | .38 | .41 (.26–1.56) | |
| Hypocomplementaemia | 21/25 (84%) | 89/100 (89%) | .49 | 1.54 (.45–5.32) | |
| Deceased | 11/25 (44%) | 10/68 (15%) | .005 | 4.56 (1.62–12.85) |
SLE: Systemic Lupus Erythematosus; SLEDAI: Systemic Lupus Erythematosus Disease Activity Index.
Given the infrequency of this complication, most knowledge regarding this entity is based on the report of cases and series from different centres like those described by Martinez-Martinez and Abdu-Mendoza, in their 2014 review (Table 4 is taken from this review, with the authors’ permission).10
Demographic characteristics of the difference series. Taken from Martinez-Martinez and Abud-Mendoza.3 Report on DAH cases published in the literature.
| Authors, year of publication | country | Number of episodes | frequency | women | Age (mean) | SLE evolution | Mean drop in haemoglobin (g/dl) |
|---|---|---|---|---|---|---|---|
| Araujo et al., 201221 | Brazil | 28 JSLE 13 SLEA 15 | 1.6% | JSLE 77% | JSLE 15.3 | JSLE 2.6y | JSLE 2.9 |
| SLEA 87% | SLEA28.7 | SLEA 5.6y | SLEA 5.5 | ||||
| Martinez-Martinez y Abud-Mendoza., 201122 | Mexico | 29 | 9% | 75.9% | 25.1 | 1.5y | 3.4 |
| Kowk et al., 201116 | South Korea | 21 | 1.4% | 90.5% | 29.7 | 5.4y | 2.1 |
| Shen et al., 201017 | China | 29 | 1.4% | 86.2% | 31 | 42m | 3.2 (median) |
| Rojas-Serrano et al., 200811 | Mexico | 14 | .6% | 96.8% | 22.4 | – | – |
| Cañas et al., 200712 | Colombia | 7 | 5.7% | 71.4% | 24.3 | 15.7m | – |
| Badsha et al., 200419 | Singapore | 22 | 1.5% | 91% | 31.6 | .96y | 3.2 |
| Chang et al.,200213 | Taiwan | 8 | .5% | 100% | 32.5 | 36m | 3.0 (median) |
| Lee et al., 200123 | Korea | 9 | – | 100% | 26 | 2m | 1.9 (median) |
| Santos-Ocampo et al., 200024 | U.S.A. | 11 | 1% | 81.8% | 31.1 | 4.5y | – |
| Lee et al.,200014 | Korea | 6 | – | 83.3% | 28 | 6m | 2.1 |
| Liu et al., 199825 | Taiwan | 13 | 4.3% | 92.3% | 26 | 23m | 2.4 |
| Zamora et al., 199720 | U.S.A. | 19 | 3.7% | 68.4% | 27 | 31m | 7.1%ht |
| Koh et al.,199726 | Singapore | 10 | – | 80% | 25 | 21.5m | – |
| Barile et al., 19979 | Mexico | 34 | 5.4% | 94.1% | 34.5 | 14.1y | – |
| Schwab et al., 199327 | U.S.A. | 8 | – | 75% | 37.9 | 2.3y | – |
| Abud-Mendoza et al.,19858 | Mexico | 12 | 1.6% | 100% | 25 | 24m | – |
| Mintz et al., 197815 | Mexico | 7 | – | 100% | 30 | 3.2y | – |
−: not informed; ht: haematocrit; JSLE: juvenile SLE; m: months; SLEA: SLE adults; y: years.
In this study 306 patients were assessed, diagnosed with SLE, 25 of them (8.2%) presented with 29 episode of DAH. The prevalence of this complication varies depending on the series, from .6%11 to 5.7%.12
We found there were series which only showed involvement in women.8,13–15 In this study 88% of the patients were women, with a mean age at presentation of 33 years, coinciding with the studies by Kwok et al.16 and Shen et al.17 However, in the series by Quintana et al. there was a lower percentage of women (64.7%) with a lower age of incidence (28 years).18
Regarding the appearance of DAH over the course of the disease, this is highly variable, and there are series which report the event from two months14 to 14 years9 after onset of SLE, and in fact a fairly large percentage may start as DAH, hindering diagnosis. In the analysed case studies it was found that haemorrhaging presented during the course of the disease after 63 months from onset with a CI of 33–93, whilst in 8/25 (32%) patients this came about at the onset of the disease, similarly to that reported by Martinez-Martinez et al.19
Studies show that DAH is a complication which usually occurs in the context of a baseline active disease (SLEDAI mean above 12 points), with the series mean of SLEDAI of 16.8 CI (12–21), where the kidney was the main organ which contributed to the scoring (similar to that described in most of the series). It is notable that Humeira et al.20 reported an increase of the SLEDAI one month prior to the event.
It became obvious that organ compromise prior to the episode in the presented series (n = 25) was: renal (69%), mucocutaneous (62%), articular (48%), serous (48%), haematological (38%), vascular (10%) and psychiatric (3%). In this sense Kowk et al.16 made a univariate analysis reporting greater risk of development of DAH in patients with serositis, neuropsychiatric lupus, SLEDAI over 10, nephritis and pulmonary hypertension and, through multivariate analysis, those of statistical significance were the neuropsychiatric SLE and the SLEDAI above 10, also demonstrated in the meta-analysis by Xu et al.21 When we looked for predictive factors of DAH in our patients we found that SLEDAI above 10 points presented with a probability of being 2.68 times likely to present. Also, we found the need for MRS and the presence of renal failure increased the probability of mortality by 20.4 and 6.93, respectively.
The clinical symptoms are highly suggestive in the majority of cases, and progress to respiratory insufficiently with a need for MRS, although there are isolated reports of asymptomatic pulmonary haemorrhaging, which may even be severe.8 None of our patients presented with this. Regarding one of the principal symptoms, haemoptysis, most of the series reported approximately 50% as the form of presentation of symptoms, similar to that found in the presented series.
Regarding complementary methods, all patients presented with a fall in haemoglobin above two points and haemoglobin under 12 g/dL; hypoxia and hypocomplementaemia in the majority of them and changes to renal function in half of them.
Some series reported a statistically significant association with thrombocytopenia with DAH, either as a predictor of the event in patients with SLE16,22 or in the DAH episode with the highest mortality.23 In our case studies we found thrombocytopenia in 31% of patients in their mild to moderate majority (in three it was severe with under 50,000) with no statistical relevance in episode (mortality) or prior to this (DAH predictor); this indicated to us that thrombocytopenia helped to contribute to the SLEDAI more than as an independent DAH factor.
All patients presented with imaging compromise in radiography and/or CAT, with similar findings to these of Ortiz et al.4 where DAH could be found without any imaging interpretation. In reality, no pathognomonic, clinical, analytical or imaging data exist for this entity and there are a considerable number of pathologies which may mimic this condition, including heart failure, pulmonary thromboembolism, lupus pneumonitis24 and other causes of diffuse alveolar damage, to mention just a few. Furthermore, infectious agents may be simple colonizers of the airways, triggers, or the causing agents of the condition, and we therefore believe that bronchioalveolar lavage (BAL) plays an essential role in early diagnosis of DAH and in ruling out infections.19 In our series BAL was practiced on all patients except one, who was intubated on hospital admittance, with the mean of siderophages being found in 35%; six with a positive culture who received antibiotic therapy adjusted to the germ as part of the initial treatment, similar to that reported in the liaterature.25
The average diagnostic delay was 4.6 days with a CI (2.5–6.7). Despite a more early and intensive search, due to suspicion of DAH in these patients, we noted that prolonging the series previously reported by this service6 did not vary delayed diagnosis.
In most series a combination of endovenous steroid pulses (1 g/day for three days methylprednisolone) with other immunodepressant therapies such as cyclophosphamide, plasmapheresis, and to a lesser extent, Rituximab was used. The dose and combination of therapies was based on case reports. Kim et al.26 reported a variation of mortality regarding treatment in their series, with higher survival in patients who received cyclophosphamide. In this series, all patients received pulse methylprednisolone, together with cyclophosphamide in 83% of them, except one who died on admittance to the intensive care unit. This was similar to that observed in a recently published series by Quintana et al.18
Among the most common complications observed were infections requiring dialysis and MRS. In total 11 (38%) patients died, mainly due to pulmonary haemorrhaging. This is a higher percentage to that reported in other series;7,11,22,27 possibly the variability in mortality is due to the heterogeneity of the patients, their severity, the diagnostic and therapeutic approach. It is of note that we have no evidence of greater mortality in those patient with positive cultures on hospital admittance compared to those who did not have them (p = 1.0 – OR = .78), similarly to the study by Martínez-Martínez et al.23
ConclusionDAH in patients with SLE is a rare complication but it always requires early diagnosis and intensive treatment to optimise survival.
Coinciding with some of the series, the need for mechanical ventilation and renal compromise results in greater, statistically significant mortality, with a higher probability of mortality.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Reibaldi AA, Sager L, Calvo R, Ortiz A, Roverano S, Paira S, et al. Hemorragia alveolar difusa en pacientes con lupus eritematoso sistémico. Reumatol Clin. 2022;18:84–90.