Diffuse alveolar hemorrhage (DAH) in patients with systemic lupus erythematosus is a rare but potentially fatal condition. Although the pathogenesis of this condition is unknown, high disease activity is the main characteristic; moreover, histopathology in some studies showed alveolar immune complex deposits and capillaritis. Clinical features of DAH include dyspnea, a drop in hemoglobin, and diffuse radiographic alveolar images, with or without hemoptysis. Factors associated with mortality include mechanical ventilation, renal failure, and infections. Bacterial infections have been reported frequently in patients with DAH, and also invasive fungal infections including aspergillosis. DAH treatment is based on high dose methylprednisolone; other accepted therapies include cyclophosphamide (controversial), plasmapheresis, immunoglobulin and rituximab.
La hemorragia alveolar difusa (HAD) es una manifestación rara pero potencialmente fatal en pacientes con lupus eritematoso sistémico (LES). La patogenia de esta manifestación es desconocida, aunque los pacientes se presentan con datos clínicos de actividad del LES en el momento de la hemorragia; estudios de histopatología han implicado depósitos de complejos inmunes e infiltrado celular (capilaritis). El cuadro clínico clásico de la enfermedad consiste en disnea, descenso en la hemoglobina e imágenes radiográficas alveolares difusas habitualmente, con o sin la presencia de hemoptisis. Se han identificado diversos factores asociados a mortalidad, entre los que se encuentran la ventilación mecánica, falla renal e infecciones; estas últimas se han descrito como frecuentes en diversas series, aunque principalmente son bacterianas, también pueden observarse infecciones fúngicas invasivas como aspergilosis. El tratamiento de la HAD se ha basado en pulsos de metilprednisolona; pueden ser útiles también, ciclofosfamida (uso controversial), plasmaféresis, inmunoglobulina y rituximab.
It's been over 100 years since Osler's description of a patient with erythema, and assumed pulmonary hemorrhage, who presented with systemic lupus erythematosus (SLE).1 DAH, today, remains one of the most devastating complications in patients with SLE; having a high mortality2 and representing a diagnostic and therapeutic challenge for the rheumatologist.
The frequency of the disease varies depending on the series consulted, from 0.63 to 5.4%4 cohorts of lupus and from 0.55 to 9%2 of hospital admissions to 5.7% of the internments to intensive care6 and 12.3% of autopsies.7 As in SLE, the frequency is higher in women and, although most of the case series reported that pulmonary hemorrhage occurs early, the mean or median progression of SLE at the time of DAH goes from 6 months8 to 14.1 years4 (Table 1).
Selected Series’ Demographic Characteristics.
| Authors, year of publication | Country | No. episodes | Frequency | Females | Age | Evolution SLE | Decrease in hemoglobin |
| Araujo et al., 20129 | Brazil | 28JSLE: 13 ASLE: 15 | 1.6%a | JSLE: 77%ASLE: 87% | JSLE 15.3dASLE: 28.7d | JSLE 2.6 adASLE: 5.6 ad | JSLE: 2.9g/dLdASLE: 5.5g/dLd |
| Martínez-Martínez and Abud-Mendoza, 20112 | Mexico | 29 | 9%b | 75.9% | 25.1d | 1.5 ad | 3.4g/dLd |
| Kwok et al., 201110 | South Korea | 21 | 1.4%b | 90.5% | 29.7d | 5.4 ad | 2.1g/dLd |
| Shen et al., 201011 | China | 29 | 1.4%b | 86.2% | 31e | 42 me | 32g/Le |
| Rojas-Serrano et al., 20083 | Mexico | 14 | 0.6%a | 92.8% | 22.4d | – | – |
| Cañas et al., 20076 | Colombia | 7 | 5.7%c | 71.4% | 24.3d | 15.7 md | – |
| Badsha et al., 200412 | Singapore | 22 | 1.5% | 91% | 31.6d | 0.96e | 3.2g/dLd |
| Chang et al., 20025 | Taiwan | 8 | 0.5%b | 100% | 32.5e | 36 me | 3.0g/dLe |
| Lee et al., 200113 | Korea | 9 | – | 100% | 26e | 2me | 1.9g/dLe |
| Santos-Ocampo et al., 200014 | EE. UU. | 1 | 1%b | 81.8% | 31.1d | 4.5 ad | - |
| Lee et al., 20008 | Korea | 6 | – | 83.3% | 28d | 6 md | 2.1g/dLd |
| Liu et al., 199815 | Taiwan | 13 | 4.3%b | 92.3% | 26d | 23 md | 2.4g/dLd |
| Zamora et al., 199716 | USA | 19 | 3.7%b | 68.4% | 27e | 31 me | 7.1% Htd |
| Koh et al., 199717 | Singapore | 10 | – | 80% | 25e | 21.5 md | – |
| Barile et al., 19974 | Mexico | 34 | 5.4%b | 94.1% | 34.5d | 14.1 ad | – |
| Schwab et al., 199318 | USA | 8 | – | 75% | 37.9d | 2.3 ad | – |
| Abud-Mendoza et al., 198519 | Mexico | 12 | 1.6%b | 100% | 25d | 24 m | – |
| Mintz et al. July 1978 | Mexico | 7 | – | 100% | 30 | 3.2 a | – |
In the series of Lee et al.,8 the data was extracted from 6 patients with lupus and in 10 patients with DAH due to different causes.
y, years; Ht, hematocrit; SLE, systemic lupus erythematosus; ASLE, adult-onset SLE; JSLE, Juvenile-onset SLE; m, months; –, not reported.
The knowledge we have of this deadly association is based on reports and case series; Table 1 shows the main series; most are from Asia and Latin America, particularly Mexico.
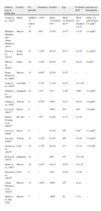
Pathogenesis and Risk FactorsActive disease is part of the DAH associated with SLE; as shown in Fig. 1, the mean or median of disease activity is high (greater than or equal to 12), indicating that the activity of SLE can be a trigger, or at least associated with DAH (Fig. 1).
Mean 2,10,11,3 or median 5,9,12 of disease activity as measured by the various scales (SLEDAI 2K2 {3 or SLEDAI)}.3,5,9–12 The first author, year and reference, in parenthesis, are shown. Data extracted from each article.
Additionally, the different series highlight the importance of disease activity of SLE by exposing the high frequency of nephritis, arthritis and neuropsychiatric disorders associated; for example, lupus nephritis is reported by the majority of studies in more than 70% of patients and in whom, very often, the histopathology was compatible with proliferative types (Table 2).
Kidney, Joint and Neuropsychiatric Manifestations in Patients With DAH Described by Different Series.
| Authors, year | Nephritis | Arthritis | Lupus NP |
| Araujo et al., 20129 | JSLE: 77%ASLE: 80% | JSLE: 69%ASLE: 73% | JSLE: 15%ASLE: 20% |
| Martínez-Martínez and Abud-Mendoza, 20112 | 100% | 75.9% | 24.1% |
| Kwok et al., 201110 | 76.1% | 33.3% | 47.6% |
| Shen et al., 201011 | 90% | – | – |
| Cañas et al., 20076 | 71.4% | 57.1% | 28.6% |
| Badsha et al., 200412 | 77.3% | 68.2% | 31.8% |
| Chang et al., 20025 | 100% | 25% | 37.5% |
| Lee et al., 200113 | 100% | 33.3% | 22.2% |
| Santos-Ocampo et al., 200014 | 70% | 10% | 10% |
| Lee et al., 20008 | 83.3% | – | 16.7% |
| Liu et al., 199815 | 100% | 15.4% | 61.5% |
| Zamora et al., 199716 | 93% | 15.8% | 42.1% |
| Barile et al., 19974 | 32.4% | 44.1% | 14.7% |
| Abud-Mendoza et al., 198519 | 66.6% | 16.6% | 58.3% |
ASLE, adult-onset SLE; JSLE, Juvenile-onset SLE; NP, neuropsychiatric; –, not reported.
Immune complexes have been described in patients with DAH associated with SLE. Hughson et al.,20 made a compilation of the literature of 20 cases of DAH in SLE patients, 15 of which showed the so-called soft bleeding, the remaining 5 DAH had capillaritis; additional Immune complexes were identified in the alveolar wall in 11/15 cases of patients with soft hemorrhage and 3/5 cases of capillaritis. In the same study, the authors describe the similarity between the vascular pathology observed in the soft hemorrhage with renal microangiopathy in lupus nephritis.20
Although the above may indicate the involvement of immune complexes as an expression of disease activity in its pathogenesis, Haupt et al.,21 study of 120 autopsies of patients with SLE, in the pursuit of alternative explanations, reported 29 patients with DAH and in only 2/29 (6.9%) there was no other factor to explain the DAH; in 5/29 (17.2%) there was evidence of aspiration as an associated factor and in 7/29 (24.1%), congestive heart failure, 9/29 (31%) presented infection and 6/29 (20.7%) renal insufficiency. We consider that, in addition to the activity of the disease, there may be other factors or conditions that favor DAH, as aforementioned.
Infections are common in SLE associated with DAH and they deserve a special section within this review.
It is reported that DAH is more common in winter2; although the cause of this fact is unknown, however, there are symptoms exacerbated by cold conditions, including epistaxis and hemoptysis,22,23 to mention an association of cold and respiratory tract bleeding.
Little is known about the type of immune response that triggers DAH in patients with SLE. In a model of DAH pristane-induced SLE in susceptible mice, the involvement of the innate immune response has been shown; severity or recovery from the insult (DAH) is dependent on adaptive immunity with significant participation of B24 cells. In this mouse model of SLE and pulmonary hemorrhage, hemorrhage is preceded by infiltration of macrophages and neutrophils24; although, on the other hand, immune deposits have not been demonstrated in this model.25
Risk factors for the development of DAH in SLE patients have been poorly described by the different series. Liu et al. report that 4 of 13 patients developed DAH the third day after treatment initiation15 plus 3 patients received cyclophosphamide in the previous month. In an analysis of 21 patients with lupus and DAH, and 83 controls without lupus and DAH, Kwok et al.,10 in a univariate analysis, reported that patients with DAH more frequently developed neuropsychiatric lupus, serositis, SLEDAI>10 nephritis and pulmonary hypertension;{AQ: for edit} in the multivariate analysis, the coexistence of neuropsychiatric lupus and SLEDAI>10 is considered an independent factor associated with the development of DAH. In this same study, an association with the development of DAH autoantibodies was found. DAH can be the first manifestation of SLE, which probably suggests that it represents a manifestation of SLE activity. Badsha et al. report a significant increase in the activity of the disease in the month prior to the development of DAH.12
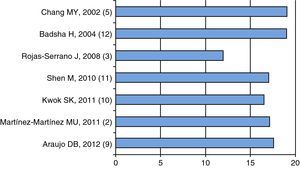
Clinical FeaturesInclusion criteria of patients for the different series presented here include dyspnea, decrease in hemoglobin, pulmonary radiographic images and hemoptysis; all these features are almost always present in patients with DAH and SLE; however, hemoptysis may not be present in more than 50% of patients, as shown in Fig. 2.
Pulmonary radiological images have been described as diffuse bilateral alveolar infiltrates in most series6,26; some have reported alveolar-interstitial infiltrates,12,14 which, in up to 20%, may be unilateral or patchy14 from 3313 to 42%.16 The resolution of radiographic infiltrates is reported by Schwab et al. in 72h.18 High-resolution CT is more sensitive than conventional radiography in detecting DAH.27
In terms of lung biopsy, some of the first studies by Myers and Katzenstein, who describe small vessel vasculitis or microangitis in 4 patients with lupus highlight the characteristic expression of DAH in SLE and immune complex deposits28. Currently, it is known that not all patients with DAH capillaritis have immune complexes associated with SLE20; e.g. Zamora et al.,16 reported capillaritis in 8 of 10 cases with biopsy (80%); one patient with DAH had diffuse alveolar damage and another infectious pneumonia. We reiterate that many patients with DAH in SLE described in the literature are reported with “soft bleeding” or without capillaritis.12
The diffusion of carbon monoxide is diagnostically useful in DAH when it is increased, a fact which occurs in 91% of patients reported by Badsha et al.12; meanwhile, Koh et al. reported this17 in 4 of 5 patients. Note that this study implies the difficulty or impossibility of its performance when patients are unstable or on mechanical ventilation, as frequently occurring in DAH.
Bronchoalveolar lavage is useful in determining the occurrence of infection3 plus it serves to identify hemosiderin-laden macrophages.29
The profile of autoantibodies including anti-DNA, lupus anticoagulant, anticardiolipin, anti-beta2-GP1, anti-Sm, anti-Ro, anti-La and anti-RNP seems no different in patients with DAH compared with SLE patients without DAH.10
Described recurrences of the disease occur in varying degrees, so a prior episode of DAH, even after receiving adequate treatment, does not preclude a new episode.2,6,9,13–16,30
Patients who survive may have alterations in respiratory function tests.31
Factors Associated With MortalitySeveral case series have tried to highlight the factors associated with mortality; the main condition associated with mortality2,10,13,16 has been mechanical ventilation and other factors are associated with the severity of the APACHE II index,25 such as azotemia2,12 and infectious processes associated with DAH.10,13,16
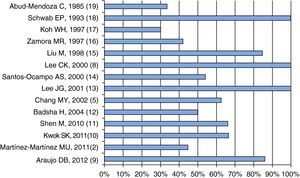
As shown in Fig. 3, mortality seems to have decreased over time; on average, it is about 50%, although there are series like Santos-Ocampo et al.,14 who reported a mortality rate of 0%. In this series, only 36% required mechanical ventilation, which is a factor associated with mortality, and other series with higher mortality report higher frequency of mechanical ventilation.2,10
With mortality we also have to evaluate the treatment received, mentioned later in this review.
InfectionsInfections have been reported by different series as an important factor associated with DAH and, in these patients with SLE and DAH, it reaches almost 60%.2,3,10,11 Bacterial infections have been reported, including Pseudomonas sp.2,5,11,3,8,16,18,32 and Staphylococcus aureus, cytomegalovirus23 infection and fungal infections, especially Aspergillus.2,11,13,16 It is difficult to separate whether the infections occurred at the time of diagnosis of pulmonary hemorrhage or after this complication. Rojas-Serrano et al.,3 reported 14 events of DAH in SLE, which were evaluated during the first 48h with bronchoscopy and bronchoalveolar lavage, finding infection in 57%; the infectious agents were Pseudomonas aeruginosa and Aspergillus fumigatus, which allowed to confirm the presence of infection in the diagnosis of pulmonary hemorrhage, leading to the observation that it could be a precipitating or contributing factor.
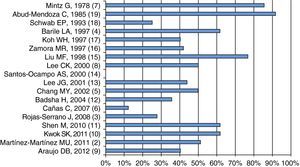
TreatmentTable 3 shows the main treatments used in the different series, of which 3 are the most frequently used: methylprednisolone, cyclophosphamide and plasmapheresis. Antibiotics were included empirically in several series.23
Treatments Used in the Different Series.
| Authors, year | mPDN | Cyclophosphamide | Plasmapheresis |
| Araujo et al., 20129 | JSLE: 100%ASLE: 100% | JSLE: 69%ASLE: 47% | JSLE: 15%ASLE: 20% |
| Martínez-Martínez and Abud-Mendoza, 20112 | 100% | 58.6% | OR |
| Kwok et al., 201110 | 95.2% | 38.1% | 66.6% |
| Shen et al., 201011 | 79.3% | 70.0% | 10.3% |
| Rojas-Serrano et al., 20083 | 57.1% | 7.1% | – |
| Cañas et al., 20076 | 100% | 100% | 57.1% |
| Badsha et al., 200412 | 86.4% | 86.4% | 50% |
| Chang et al., 20025 | 100% | 12.5% | 37.5% |
| Lee et al., 200113 | 66.7% | 33.3% | OR |
| Santos-Ocampo et al., 200014 | 81.8% | 70% | 45.5% |
| Lee et al., 20008 | 100% | 33.3% | 83.3% |
| Liu et al., 199815 | 76.9% | 15.4% | – |
| Zamora et al., 199716 | 94.7% | 52.6% | 63.2% |
| Koh et al., 199717 | 80% | 70% | 40% |
| Barile et al., 19974 | 73.5% | 5.9% | 5.9% |
| Schwab et al., 199318 | 87.5% | 62.5% | – |
| Abud-Mendoza et al., 198519 | 25% | 8.3% | – |
ASLE, adult-onset SLE; JSLE, Juvenile-onset SLE; mPDN, methylprednisolone pulses; –, not reported.
The use of high-dose intravenous “pulses” of methylprednisolone is one of the most widely used treatments in SLE associated with DAH. In a retrospective study, Barile et al.,4 divided 34 patients with DAH associated with SLE into 3 groups: patients who received prednisone 1mg/kg, another “conventional” form of pulse methylprednisolone (3g in 3 days) and those who received pulse methylprednisolone until the resolution (more than 3g); survival was higher for patients receiving prolonged pulses and reduced in those which only received prednisone 1mg/kg.
The use of cyclophosphamide is controversial; while Zamora et al. reported a higher mortality in patients who received the drug,16 the series probably benefited due to the association of SLE disease activity with DAH.12 Zamora et al.,16 reported that all patients who received cyclophosphamide required mechanical ventilation, plus 6/7 patients died. DAH also presented infection, factors associated with mortality in the same study, making it unlikely that cyclophosphamide confers, by itself, an increase in mortality, as patients who received it had more factors associated with mortality.16
As shown in Table 3, plasmapheresis has been used in several series; it is difficult to assess its utility alone, since although some studies show a lower mortality of 20%, patients were treated with or without cyclophosphamide6; the same could be said for intravenous immunoglobulin, which has been used in some series, such as Shen et al.,11 and case reports.
Biological therapies for DAH in SLE may also be associated with benefits that have been reported in case reports, as in the likely benefit of rituximab33–35; although in the series by Martínez-Martínez and Abud-Mendoza this was not beneficial in 2 patients.2 Note that the mechanism of action of rituximab on autoantibodies could not occur quickly, as required in SLE associated DAH; however, other mechanisms of action of B cell depleting therapy may be involved in fast beneficial effects, such as antigen presentation and activation of T regulatory cells.24,36,37 Rituximab may be more useful to prevent recurrences.2,30
Other therapies include transplantation of umbilical cord mesenchymal cells,38 reporting a benefit in 4 patients as published by Shi et al.39 There are also reports of treatment with extracorporeal membrane oxygenation,40 factor vii41 and42 mycophenolate mofetil.
Conclusions and Future ProspectsDespite the passage of time and new therapies, DAH associated with SLE remains a diagnostic and therapeutic challenge, with high mortality; identifying factors associated with mortality and the measures and strategies to diminish or avoid it could improve the survival rate. Controlled studies or continuation of the series and a combination of cases is required to analyze and evaluate the potential benefit of current therapies, which include biological drugs.
DAH can result from joint factors such as the activity of the disease, which leads to a potentially fatal process which can be accompanied by infections, heart and renal failure, in addition to immunoregulation alterations, and they are all characteristics of SLE. We suggest that all patients with DAH be assessed and monitored from admission to rule out infectious processes and receive appropriate comprehensive therapy, with the inclusion of antibiotics and immunosuppressants, with ongoing assessments to identify factors associated with morbidity and mortality.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that this research has not performed experiments on humans or animals.
Data privacyThe authors state that patient data does not appear in this article.
Right to privacy and informed consentThe authors state that patient data does not appear in this article.
Conflicts of InterestThe authors have no conflicts of interest.
Please cite this article as: Martínez-Martínez MU, Abud-Mendoza C. Hemorragia alveolar difusa en pacientes con lupus eritematoso sistémico. Manifestaciones clínicas, tratamiento y pronóstico. Reumatol Clin. 2014;10:248–253.