To analyze the resource utilization in rheumatoid arthritis (RA) patients and predictive factors in and patients treated with biological drugs and biologic-naïve.
MethodsA cross-sectional study was performed in a sample including all regions and hospitals throughout the country. Sociodemographic data, disease activity parameters and treatment data were obtained. Resource utilization for two years of study was recorded and we made costs imputation. Correlation analyzes were performed on all RA patients and those treated with biological and biological naïve, to estimate the differences in resource utilization. Factors associated with increased resources utilization (costs) attending to treatment was analyzed by linear regression models.
ResultsWe included 1.095 RA patients, 26% male, mean age of 62±14 years. Mean of direct medical costs per patient was €24,291±€45,382. Excluding biological drugs, the average cost per patient was €3742±€3711. After adjustment, factors associated with direct medical costs for all RA patients were biologic drugs (P=.02) and disease activity (P=.004). In the biologic-naïve group, the predictor of direct medical costs was comorbidity (P<.001). In the biologic treatment group predictors were follow-up length of the disease (P=.04), age (P=.02) and disease activity (P=.007).
ConclusionOur data show a remarkable economic impact of RA. It is important to identify and estimate the economic impact of the disease, compare data from other geographic samples and to develop improvement strategies to reduce these costs and increase the quality of care.
Estimar los costes médicos directos en los pacientes con artritis reumatoide (AR) y los factores predictores en los pacientes tratados con fármacos biológicos y sin biológicos.
MétodosSe realizó un estudio transversal en una muestra incluyendo pacientes de toda la geografía nacional. Se obtuvieron datos sociodemográficos y de tratamiento. Se registró la utilización de recursos para los 2 años de estudio y se hizo imputación de costes. Se realizaron análisis de correlación en todos los pacientes con AR y en los tratados con y sin biológicos, para estimar las diferencias entre los grupos. Los predictores de costes se analizaron mediante modelos de regresión lineal.
ResultadosSe incluyeron 1.095 pacientes con AR, el 26% hombres, con una edad media de 62±14 años. La media de los costes médicos directos por paciente fue de 24.291±45.382€. Excluyendo los fármacos biológicos, el coste medio por paciente fue de 3.742±3.711€. Después de ajustar, los factores predictores de costes médicos directos para todos los pacientes con AR fueron los fármacos biológicos (p=0,00), la comorbilidad (p=0,00) y la edad del paciente (p=0,01). En el grupo sin biológicos, los predictores fueron la comorbilidad (p=0,00) y la edad del paciente (p=0,01). En el grupo con biológicos los predictores fueron el sexo del paciente (p=0,03) y la actividad de la enfermedad (p=0,02).
ConclusiónLos datos muestran un notable impacto económico de la AR. Es importante identificar y estimar los factores asociados a mayor coste para desarrollar estrategias de reducción de costes y aumentar la calidad de la atención.
Rheumatoid arthritis (RA) is a chronic inflammatory disease with a prevalence of approximately 0.8 (0.3–1.0) per 100 adults. The peak in the onset of the disorder ranges between 55 and 64 years of age.1–3 As a consequence of this condition, RA patients consume a great deal of health care resources, and the costs are high. Given the cost of treatment and the loss in productivity, this disease has a great impact on society. Studies on the costs of the disease conducted in Europe and the United States estimate a figure of around €4000–€6000 per patient per year.4–6
In Spain, the management of inflammatory rheumatic diseases has undergone marked advances due to several factors such as the availability of new treatments, a deeper understanding of the processes and progress in the care of the rheumatic patient.
The increase in the use of biological agents in routine rheumatology practice has raised the direct costs 3 to 6-fold in several European countries.7,8 However, changes in the patterns of treatment have achieved a decrease in the mean disease activity (Disease Activity Score 28 joints [DAS28]), hospital stays, lost work days and work disability,9 thus resulting in a reduction in the indirect costs related to the disease.
In a number of studies performed in the 1980s and 1990s,10–13 the major medical expense was hospital admission, which, in those articles, represented a third of the cost. In later studies, the greatest cost corresponded to the drugs prescribed.14 This change was associated with a growing percentage of patients treated with biological agents.
The purpose of the study entitled “Variability in the management of rheumatoid arthritis and spondyloarthritis in Spain” (EMAR-II) was to describe variability in terms of health care resource consumption and the use of techniques and treatments and, thus, made it possible to define these changes in this country. Hospitals of different autonomous communities participated in the study, which had a duration of 2 years (Appendix A). This enabled us to recruit a representative cohort of the disease in Spain.
There are few studies on the costs of RA in the biological era, and those that exist have been conducted in settings in which these agents were still little employed. Thus, the objective of our report was to estimate the direct medical costs corresponding to the EMAR-II cohort, analyze factors that may be predictive of those costs and evaluate the predictive factors in 2 distinct subpopulations: patients who were treated with biological agents during the study period and those who did not receive them.
Material and MethodsDesign, Patient Selection and Data AcquisitionThe EMAR-II study (2009–2010) was a cross-sectional study designed to analyze variability in the management of patients with RA in Spain. We established contact with 100 hospitals, 46 of which agreed to participate, whereas 54 chose not to. The sample was composed of the medical records of the RA patients who were being seen in the rheumatology departments of the Spanish hospitals and had had at least one visit with their rheumatologist over the 2 years prior to the date of the initiation of the study. In order to recruit at the different participating centers, the random sampling was stratified by autonomous community, with the sampling done in 2 stages, by hospital (units of the first stage) and by patients (units of the second stage). To prevent the lack of representativeness associated with the homogeneity of the different sized units of the first stage, the sampling in that stage was done with a probability proportional to size, whereas, in the second stage, we performed randomized equiprobable sampling of the patients from each center. The smallest autonomous communities were grouped with the largest to ensure that all be represented. The data from the 2 preceding years were extracted from the patients’ health records and copied on standardized forms, the same for every case, and a handbook was created with instructions on how to fill them out. The data were then introduced into an electronic database designed specifically for this project.
Study SubjectsRA patients selected for EMAR-II, which had a total of 1272 participants. Variables: the following variables were included: (a) sociodemographic variables: patient age and gender; (b) clinical data: time since RA onset, comorbidity, acute phase reactants (erythrocyte sedimentation rate [ESR], C-reactive protein [CRP] or others), composite disease activity indices (DAS, Simplified Disease Activity Index [SDAI] or others), visual analog scales (VAS) with the highest and lowest physician and patient global assessments, evaluation of the functional status in terms of disease activity (functional status according to the American College of Rheumatology and the Health Assessment Questionnaire), and a variable we refer to as disease activity (generated for this study), composed of a DAS28 in active disease (≥3.2) in 2 visits or a high ESR (≥50mm/h) in 2 measurements or a high subjective physician assessment (SPA) (≥moderate level) in 2 visits, and (c) the cost variables assigned to health care resource consumption: number of medical visits to other services, number and types of nonmedical visits, RA-related hospital admissions, orthopedic surgery, ancillary studies (laboratory, diagnostic imaging, others), arthrocentesis, drugs (nonsteroidal anti-inflammatory drugs [NSAID], glucocorticoids, disease-modifying antirheumatic drugs [DMARD], biological agents).
Data SourcesThe direct costs in this study are defined as costs for the Spanish social security system, the primary payer of these expenditures in Spain. The Spanish health care system offers universal coverage financed by taxes that operates mainly within the public sector. The services are free at the point of care, with the exception of the drugs prescribed to people under the age of 65 years, who, in most cases, have to pay 40% of the retail price.
Using the EMAR-II database, we generated a specific database for this study that included data on the RA patients and the costs were allocated. Given the large costs involved in the study, we needed to employ several data sources. These were provided by the official gazettes of the Spanish autonomous communities and were published by their health departments or by the Ministry of Health, the Spanish Agency for Consumer Affairs, the Spanish Institute of Health, the Institute of Health Information or other sources, like the Soikos health cost database, version 2.2.15–23 All of the costs are expressed in 2010 euros.
Statistical AnalysisWe describe the sociodemographic, clinical and cost variables of the sample utilizing frequency distribution, mean and standard deviation. As the data were not normally distributed, the dependent variable—costs—was transformed to approximate normality. This descriptive analysis was also performed for the group of patients who took biological agents and for those who did not receive those drugs during the study period. The differences between the groups in terms of direct medical costs were estimated using contingency tables and Student's t test.
Bivariate and multivariate analyses were carried out in order to estimate the possible predictive factors of the direct medical costs in the entire sample employing linear regression models. We subsequently performed a secondary analysis using linear regression methods to estimate the differences and the predictors of the direct medical costs in the group that received biological agents and in the group that did not. The data shown correspond to a study period of 2 years. The analyses were performed with the Stata 10.0 statistical software package (Stata Corp, College Station, TX, United States).
ResultsThe final study sample consisted of a total of 1095 patients for whom all of the data on medical costs were available.
In all, 26% of the patients included in the study were men. The mean age was 62±14 years. With respect to the clinical characteristics of the sample, the duration of the disease was 10.2±8.9 years. Overall, 49.8% of the patients had some type of associated comorbidity, distributed as follows: nodules (12.16%), Sjögren's syndrome (10.53%), carpal tunnel syndrome (6.82%), Raynaud's phenomenon (2.5%), C1–C2 subluxation (2.5%), pulmonary fibrosis (2.3%), pleuritis (1.4%), vasculitis (0.9%), scleritis (0.9%), Felty's syndrome (0.4%) and amyloidosis (0.15%). The highest DAS28 score during the study period was recorded in patients with low activity (DAS≤3.2) in 27.76%, moderate activity (DAS 3.2–5.1) in 45% and high activity (DAS>5.1) in 27.24%. The mean minimum ESR was 15.26±14.10 and the maximum values were 38.30±27.13. The SPA had minimum values of 15.26±14.10 and the maximum values were 38.30±27.13. Lastly, for the disease activity variable generated for this study (a combination of DAS28 in active disease [≥3.2] in 2 visits or an elevated ESR [≥50mm/h] in 2 measurements or an elevated SPA [≥moderate level] in 2 visits), we found that 26.14% of the patients had high activity and 73.86% of the patients had low activity.
In all, 67.3% of the participants took NSAID during the study period and 67.6% received corticosteroids. Overall, 20.7% of the patients had received 2 or more DMARD simultaneously over the preceding 2 years. The DMARD most widely administered during the study period was methotrexate (59.6%), followed by leflunomide (22.1%), antimalarial drugs (12.2%) and sulfasalazine (3.1%). Altogether, 36.95% of the sample received some type of biological therapy during the study period. The agents most widely used were adalimumab (27.3%), infliximab (20.5%); etanercept 50mg (19.9%), etanercept 25mg (12.2%) and rituximab (7.6%).
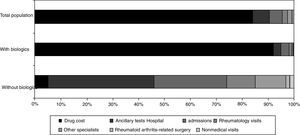
The costs were calculated using the monetary value assigned to the health services according to the different data sources consulted. The total direct medical cost per patient was €24,291±45,382 throughout the 2 years of the study. The total expenditure for drugs (DMARD, biological agents, NSAID, corticosteroids and analgesics) was €19,567±43,560. If we exclude the cost of biological drugs, the mean cost per patient was €3742±3711. The distribution of the costs is shown in Figure 1. There was a statistically significant gender-related difference, with an average cost of €17,330±1993 for men and of €26,814±1720 for women (P=.002).
With respect to differences between the groups, the patients who received biological agents had a significantly higher cost in relation to the following variables: visits to the rheumatologist; visits to the nursing service; orthopedic surgery without prosthesis; orthopedic surgery of the spine; social service counseling; psychology unit; rehabilitation services; complete blood counts; laboratory testing; sedimentation; protein electrophoresis; serology; cultures; rheumatoid factor; ESR; CRP; anti-cyclic citrullinated peptide antibodies; chest radiography; axial bone radiography; magnetic resonance imaging; Mantoux test; pulmonary function tests; arthrocentesis; and hospital admission.
There were no differences in the costs for the other variables evaluated. Although it was not statistically significant, the patients who received biological agents were less costly in terms of visits to other specialists, tests for human leukocyte antigen B27, abdominal radiographs, certain other types of radiographs and abdominal ultrasound.
Bivariate linear regression analysis showed that the variables associated with direct medical costs were patient sex—with higher costs for women (P=.002), patient age (P=.00), disease duration (P=.00), disease activity (P=.00) and treatment with biological drugs (P=.00).
Multivariate regression analysis was subsequently performed with all of the variables that had been significant in the bivariate analysis, which were adjusted for the sociodemographic variables (Table 1). In the analysis of the predictors of medical costs for the sample as a whole, the predictive factors were biological agents (P=.00), comorbidity (P=.00) and patient age (P=.01).
Multiple Linear Regression Analysis of the Direct Medical Costs in the Population of Rheumatoid Arthritis Patients.
| Patients with rheumatoid arthritis | ||||
|---|---|---|---|---|
| β coefficient | Standard deviation | t | P | |
| Biological agents | 1.23 | 0.02 | 50.80 | .000 |
| Comorbidity | 0.07 | 0.01 | 6.77 | .000 |
| Disease duration | 0.0009 | 0.001 | 0.72 | .474 |
| Patient age | −0.002 | 0.0008 | −2.50 | .012 |
| Patient sex | 0.04 | 0.025 | 1.59 | .112 |
| Disease activity | 0.01 | 0.025 | 0.64 | .524 |
| Constant | 3.40 | 0.05 | 59.61 | .000 |
Statistically significant values, that is, those with P<.05, are in boldface.
A bivariate model was utilized in the analysis by subgroup. For the group of patients who did not receive biological drugs, the variables associated with costs were comorbidity (P=.00) and patient age (P=.01). For the group of patients who took biological drugs, the variables that were associated with costs were disease duration (P=.02), patient sex—with higher direct medical costs for women (P=.01)—and disease activity (P=.00).
Finally, 2 models of multivariate regression were built, one for each group of patients, including the variables that were statistically significant in the bivariate analysis. In the analysis of the direct medical cost predictors in the group of patients that did not receive biological agents during the study period, the independent predictive factors identified were comorbidity (P=.00) and patient age (P=.02) (Table 2). In the analysis of the predictors of direct medical costs in the group of patients who took biological drugs during the study period, the independent factors detected were patient sex (P=.03) and disease activity (P=.02) (Table 3).
Multiple Linear Regression Analysis of the Direct Medical Costs in Patients Who did not Receive Biological Agents.
| Patients who did not take biological agents | ||||
|---|---|---|---|---|
| β coefficient | Standard deviation | t | P | |
| Comorbidity | 0.10 | 0.01 | 9.02 | .000 |
| Disease duration | 0.0005 | 0.001 | 0.38 | .704 |
| Patient age | −0.002 | 0.0009 | −2.23 | .026 |
| Patient sex | 0.006 | 0.027 | 0.23 | .821 |
| Disease activity | −0.03 | 0.031 | −1.11 | .269 |
| Constant | 3.40 | 0.06 | 55.39 | .000 |
Statistically significant values, that is, those with P<.05, are in boldface.
Multiple Linear Regression Analysis Of The Direct Medical Costs In Patients Who Received Biological Agents.
| Patients who received biological agents | ||||
|---|---|---|---|---|
| β coefficient | Standard deviation | t | P | |
| Comorbidity | 0.009 | 0.02 | 0.45 | .654 |
| Disease duration | 0.001 | 0.002 | 0.56 | .573 |
| Patient age | −0.002 | 0.001 | −1.67 | .096 |
| Patient sex | 0.104 | 0.049 | 2.11 | .036 |
| Disease activity | 0.09 | 0.043 | 2.25 | .025 |
| Constant | 4.63 | 0.10 | 45.60 | .000 |
Statistically significant values, that is, those with P<.05, are in boldface.
This retrospective study calculates the direct costs involving the recourses utilized by the patients. The results of this report provide an extensive characterization of RA patients in Spain. Their distribution in terms of age and sex was similar to that of other published series,24 the mean age of the patients was somewhat over 60 years of age and 26% were men.
The costs (excluding biological drugs), with a mean of €3742 during the study period, were lower than those reported for patients in other studies, in which the annual cost was around €3980.25
It appears to be clear that biological agents are the bulk of these direct medical costs, although other factors have a role, like disease activity, in the case of patients taking biologicals. In individuals who do not receive these drugs, patient age and comorbidities are major predictors of direct medical costs.
There are studies that show that the increase in the cost of therapy produced by the introduction of biological agents has decelerated since 200926 since the rate of prescriptions is growing, but more sequentially. This has also resulted in the optimization of the drugs with less rigid protocols that can be better adapted to the characteristics of the patient. Given the trend toward early treatment, it is expected that the costs will stabilize over time.
It seems evident that the direct medical costs of RA patients have risen due to the introduction of new treatments, as well as to the aging of the patients, which is increasing in parallel to the age of the general population. However, although this study does not focus on indirect costs, other reports indicate that they are being compensated by reductions in other costs. In fact, a recent study shows a continuous increase in annual medical costs for patients with RA who were being treated by German rheumatologists between 2002 and 2011, caused by the growing use of biological agents. However, the rise was partially offset by the decrease in the costs of hospital admissions, sick leave and work disability, with a reduction in lost work days and fewer cases of permanent disability.27
On interpreting the results of this study, certain limitations must be taken into account. Data were collected for only 2 years and, thus, the interpretation of the findings should be done with caution. Moreover, it should be kept in mind that certain data may not be extractable from the medical records or not be documented, both of which influence the validity of the results.28 Likewise, the cost data are not exact, but rather are assertions taken at the national level. The costs per patient can be incorrectly estimated, especially because differences related to the community and center were not taken into account. It is important to point out that, because of the type of study, it was impossible to establish the indirect costs associated with work productivity and other causes. Therefore, we were not able to estimate the impact on the indirect costs that could have resulted from a better therapy management or the introduction of biological drugs, as has been seen in other studies.
In conclusion, this study presents a detailed map of the direct medical costs associated with RA patients in Spain. These data may contribute to the understanding and estimation of the economic impact of the disease, to their comparison with data from other geographic areas, to the development of strategies for the reduction of these costs and to an increase in quality of care. Along this line, recommendations on the optimization of drugs and treatment profiles adapted to the patient are of major importance, as well as the possibility of establishing nurse counseling to instruct patients and broaden their knowledge of the disease, thus reducing medical visits and unnecessary tests in many cases.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have adhered to the protocols of their centre of work on patient data publication.
Right to privacy and informed consentThe authors must have obtained the informed consent of the patients and /or subjects mentioned in the article. The author for correspondence must be in possession of this document.
FundingThe EMAR-II study was promoted by the Spanish Society of Rheumatology and financed by the pharmaceutical company AbbVie.
Conflicts of InterestThe EMAR-II study was financed by the pharmaceutical company AbbVie.
C. Escudero, N. Chozas, I. Maries, A. Fernandez, F. Medina, I. Ureña, V. Irigoyen, M. Lopez, P. Espiño, S. Manrique, E. Collantes, P. Font, D. Ruiz, M. Granados, M.J. Pozuelo, I. Moreno, J.M. Pina, R. Roselló, C. Vázquez, J. Beltrán, F.J. Manero-Ruiz, A. Pecondón, E. Giménez, F. Jimenez, J. Marzo, M. Medrano, J. Babío, T. Tinturé, S. González, C. Ordás, M.E. García, L. Espadaler, J. Fernandez, J. Fiter, A. Naranjo, S. Ojeda, J. Tornero, J.A. Piqueras, E. Júdez, C. López, J. Medina, G. Iglesias, M. Alvarez, J. Alegre, M.R. Colazo, J.L. Alonso, B. Alvárez, C. Montilla, S. Gómez, R. López, M. Sánchez, S. Castro, S. Ordóñez, D. Boquet, J. Calvet, D. de la Fuente, V. Rios, M. Nolla, A. Martínez-Cristóbal, R. Negueroles, M.L. Muñoz, J. García, F. Gamero, E. del Rincón, E. Pérez-Pampín, L. Fernandez, R. Miguélez, A.M. Ortíz, E. Vicente, S. Pérez Esteban, E. Tomero, A. Casado, M.J. Arias, E. Cuende, C. Bohorquez, J.M. Rodríguez, A. Aragón, J. García, J. Zubieta, A. Gallego, C. Martínez, I. Mateo, A. de Juanes, E. Enríquez, I. Monteagudo, F.J. López-Longo, E. Pagán, M.J. Rubira, P. Mesa, J. Galvez, E. Saiz, C. Tornero, E. Úcar, C. Rodríguez, B. González Álvarez, N. Rivera, F.X. Arasa, S. Bustabad, E. Delgado, J. Maese and R. Veroz.
Please cite this article as: Leon L, Abasolo L, Fernandez-Gutierrez B, Jover JA, Hernandez-Garcia C. Costes médicos directos y sus predictores en la cohorte “Variabilidad en el manejo de la artritis reumatoide y las espondiloartritis en España”. Reumatol Clin. 2018;14:4–8.