A retrospective economic evaluation was performed on the restriction of the use of piroxicam in Spain, a non-steroidal anti-inflammatory drug, with a proven higher risk of serious gastrointestinal complications compared to other non-steroidal anti-inflammatory drugs with the objective of putting the relevance of these activities into context.
MethodsA retrospective cost-effectiveness analysis and a budget impact analysis were performed. Costs and cases of serious gastrointestinal complications were compared in the non-intervention (use of piroxicam) and the intervention scenarios (use of other non-steroidal anti-inflammatory drugs). The cost of serious gastrointestinal complications was obtained from the Diagnosis Related Groups and the cost of non-steroidal anti-inflammatory drugs from usage data in the Spanish national health system. The risk of serious gastrointestinal complications was obtained from epidemiological studies.
ResultsThe regulatory intervention was the dominant option. In that sense, 0.81 euros per treated patient were saved, 2.75 cases of serious gastrointestinal complications were avoided per 10,000 patients and 578,608 euros were saved in total in Spain in the first year following the intervention.
ConclusionsIt is possible to perform complete economical evaluations on pharmacovigilance actions. The intervention performed by the Spanish Agency for Medicines and Medical Devices, AEMPS on piroxicam not only achieved the objective of preventing adverse drug reactions but also resulted in significant economical savings even under conservative assumptions.
Se ha realizado una evaluación económica retrospectiva de la restricción llevada a cabo en España en el uso de piroxicam, debido a un riesgo constatado de complicaciones gastrointestinales graves mayor que el de los otros antiinflamatorios no esteroideos, con el fin de poner en contexto la relevancia de las actividades de farmacovigilancia.
MétodosSe realizaron análisis de coste-efectividad y de impacto presupuestario retrospectivos. Se compararon costes y casos de complicaciones gastrointestinales graves en el escenario sin intervención (uso de piroxicam) y con intervención (uso de otros antiinflamatorios no esteroideos). Se ha obtenido el coste de complicaciones gastrointestinales graves mediante los Grupos Relacionados por el Diagnóstico del conjunto mínimo básico de datos y el de los antiinflamatorios no esteroideos con los datos de consumo en el Sistema Nacional de Salud. El riesgo de complicaciones gastrointestinales graves se estimó a partir de estudios epidemiológicos.
ResultadosLa intervención reguladora resultó la opción dominante, con un ahorro de 0,81 euros por paciente y 2,75 casos de complicaciones gastrointestinales graves evitados por 10.000 pacientes y un ahorro estimado en toda España de 578.608 euros en el primer año tras la intervención.
ConclusionesEs posible realizar evaluaciones económicas completas sobre actuaciones en farmacovigilancia. La intervención realizada por la Agencia Española de Medicamentos y Productos Sanitarios sobre piroxicam, además de cumplir con su objetivo de prevenir reacciones adversas, supuso un ahorro significativo, incluso bajo supuestos conservadores.
In September 2006, the European Medicines Agency (EMA), at the request of the European Commission, started a formal review of the benefit-risk balance of the non-steroidal anti-inflammatory drug (NSAID) piroxicam since different epidemiological studies consistently pointed out that piroxicam presented a higher risk of gastrointestinal complications than other NSAIDs. The EMA's Committee for Medicinal Products for Human Use came to the conclusion that the benefit-risk ratio of piroxicam is only favourable in very restricted conditions of use.1 As a consequence, in September 2007, the AEMPS (Agencia Española de Medicamentos y Productos Sanitarios [Spanish Agency of Medicines and Medical Devices]) restricted the systemic use of medications that contained piroxicam, as well as their prescription to hospital diagnostic, only for the symptomatic treatment of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis, but never as first line of treatment.2
Several studies3,4 have analysed, on a global basis, the financial impact of adverse reactions to medications on the health of the population. In particular, the financial and health care significance of the gastrointestinal adverse effects of NSAIDs5 is revealed by the significant amount of pharmacoeconomic studies performed in this area, some of them in Spain,6–10 designed to analyse the efficiency of introducing the use of less gastroerosive NSAIDs (specifically, those called Coxib) and, more recently, to study the efficiency of certain therapeutic strategies in the use of NSAIDs.11,12
It is safe to assume that regulatory interventions in pharmacovigilance, aimed at reducing the disease load associated to adverse reactions to drugs, produce an effective cost and disease reduction in the population in which they are applied. However, there is no literature regarding a complete economic assessment of concrete interventions in pharmacovigilance. These decisions are taken regardless of their economic consequences, and a prior pharmacoeconomic assessment is not deemed necessary or pertinent. However, in the context of the necessary effectiveness assessment of the measures taken, a subsequent assessment, including their financial impact on the health care system, may be useful to place their relevance as public health activity in context.
Therefore, the purpose of this study is to perform an economic assessment of the intervention in pharmacovigilance that took place in 2007 regarding piroxicam compared to the situation that would have occurred if the aforementioned regulatory measure had not been taken, with a time horizon of 1 year. To that effect, a cost-effectiveness analysis and a budget impact analysis have been performed.
Subjects and MethodsReference PopulationCandidates to receive piroxicam via prescription from the SNS (National Health System [Sistema Nacional de Salud]) in Spain during the years 2006–2008. Those patients for whom piroxicam continued to be indicated after the restriction of use was decided by the AEMPS were excluded.2
For the base case, an average duration of treatment with a NSAID of 20 days is considered, based on the most frequent duration of one bottle of a NSAID and, therefore, of one prescription, considering the defined daily dose (DDD) as daily average dose. The usage population-based data for these drugs in Spain show that most of the treatments with prescribed NSAIDs last less than 31 days.13
ViewpointThe viewpoint is the SNS's viewpoint, so for cost calculation only direct medical costs for the health care system are taken into account.
Intervention to Be AssessedThe regulatory intervention took place in September 2007 for pharmacovigilance reasons, consisting of the restriction of piroxicam to second line of treatment and exclusively in chronic pathology of joints, due to its increased risk of producing serious gastrointestinal complications in comparison with other NSAIDs of similar analgesic and anti-inflammatory efficacy.
Assessment of Health ResultsHealth results have been assessed in terms of clinical effectiveness, in the prevention of cases of serious gastrointestinal complications associated with the use of NSAIDs. The therapeutic efficacy of piroxicam and other alternative NSAIDs is assumed to be similar and the rest of the security profile may be considered comparable or, in any case, that piroxicam does not show any advantage with respect to the other NSAIDs.
To this end, the four NSAIDs with the highest use in Spain at that time were selected: ibuprofen, diclofenac, naproxen and aceclofenac, which altogether represent more than 85% of the consumption of NSAIDs.14 Serious gastrointestinal complications are defined as cases of perforation, obstruction or upper gastrointestinal bleeding. The risk or probability of presenting a serious gastrointestinal complication with piroxicam and with the alternative NSAIDs has been obtained through the calculation of the accumulated incidence of SGIC during the treatment period (IAtrto) as of the baseline incidence rates (IR) and the relative risks (RR) for each NSAID through the following formula: IAtrto=1−ESP(−IR×20).15 The risk of serious gastrointestinal complications is assumed to be constant throughout the treatment.
The SGIC IR in the general population not exposed to NSAIDs has been obtained from a meta-analysis of published epidemiological studies.16
From the different studies17–21 that have studied the risk of serious gastrointestinal complications associated with the NSAIDs of interest in the analysis (Table 1), the RR obtained in the meta-analysis of observational studies published more recently have been selected.21
Serious Gastrointestinal Complications Associated With Non-steroidal Anti-inflammatory Drugs. Results From Published Studies, Relative Risk (Confidence Interval of 95%).
| Source and type of studya | Piroxicam | Ibuprofen | Diclofenac | Naproxen | Aceclofenac |
|---|---|---|---|---|---|
| Henry et al. (2003) | 8.1 | 2.2 | 3.8 | 4.0 | – |
| Meta-analysis, 44 observational studies. 1985–2001, mostly in Northern Europe and USA17 | (6.2–10.4) | (1.7–2.3) | (3.0–3.8) | (3.3–4.8) | |
| Laporte et al. (2004) | 15.5 | 3.1 | 3.7 | 10.0 | 1.4 |
| Hospital case control study. 1998–2001, Spain and Italy18 | (10.0–24.2) | (2.0–4.9) | (2.6–5.4) | (5.7–17.6) | (0.6–3.3) |
| Lanas et al. (2006)b | 12.6 | 4.1 | 3.1 | 7.3 | 3.1 |
| Hospital case control study. 2001–2004. Spain19 | (7.8–20.3) | (3.1–5.3) | (2.3–4.2) | (4.7–11.4) | (2.3–4.2) |
| Coxib and traditional NSAID Trialists’ (CNT) Collaboration (2013) | – | 3.97 | 1.89 | 4.22 | – |
| Meta-analysis in clinical trials network. Up to 2009, mostly in Northern Europe and USA20 | (2.2–7.1) | (1.2–3.1) | (2.7–6.6) | ||
| Castellsague et al. (the SOS project) (2012)c | 7.4 | 1.8 | 3.3 | 4.1 | 1.4 |
| Meta-analysis, observational studies. Up to 2011, mostly in Northern Europe and USA21 | (5.2–10.6) | (1.5–2.2) | (2.8–4.0) | (3.2–5.2) | (0.6–3.1) |
Published studies and meta-analyses that were used as a base for the regulatory decision in Europe,1 and more recent meta-analyses.
The costs incurred for diagnosis and treatment of the serious gastrointestinal complications, which are assumed to always take place in the hospital environment, have been assessed. The rest of the costs that could be incurred in may be considered common costs to the compared alternatives.
The average hospital cost of a SGIC in the base case, in the SNS in Spain, in 2008, has been estimated in 2876.39 Euros. This has been obtained based on the cost of diagnosis-related groups (DRG) of the hospital discharge diagnoses from the Minimum Basic Data Set (MBDS) (DRG state rule [AP-GRD V23], year 2008). The selected DRG codes corresponding to the serious gastrointestinal complications, previously used by other authors,11 together with other DRG cost and the number of respective severity-adjusted discharges,22 were the following: 3598.75 Euros and 11,472 discharges for gastrointestinal bleeding with complications (code GRD 174); 2363.49 Euros and 16,867 discharges for gastrointestinal bleeding without complications (code GRD 175) y 3233.66 Euros and 1019 discharges for complicated peptic ulcer (code GRD 176). Moreover, cost assessment estimates for serious gastrointestinal complications in Spain published in literatures,5–8,11,23 which have been used for the sensitivity analysis (Table 2), have been reviewed.
Sources of Information for Average Cost Assessment of a Serious Gastrointestinal Complication Associated With Non-steroidal Anti-inflammatory Drugs.
| Study year, environment and source | Average cost per patient | |
|---|---|---|
| Original study | Update to 2008 | |
| Lanas (2000) | 312 654 pts. | 2559.07€ |
| Multicentre study in Spanish hospitals. 19985 | ||
| Vargas (2001) | 387 444 pts. | 2963.31€ |
| Study in two hospitals in Madrid and Barcelona. 20006 | ||
| Según et al. (2002)a | 3155.31€ | 3962.59€ |
| Reference hospital from a Health Area, 20007 | ||
| Moreno et al. (2003) | 2350.90€ | 2913.06€ |
| Costs taken from bibliography. 20018 | ||
| Lanas et al. (2005) | 2786.00€ | 3235.31€ |
| Costs taken from IASIST. 200323 | ||
| Rubio-Terrés (2010)b | 1918.00€ | 1944.85€ |
| DRG cost-Soikos 2005-B. medical costs data. v2.2. 200711 | ||
| DRG state rule (AP-GRD V23), MSSSI (2008)c | 2876.39€ | 2876.39€ |
| DRG costs. Codes 174, 175, 17622 | ||
DRG: diagnosis-related groups; MSSSI: Ministerio de Sanidad, Servicios Sociales e Igualdad (Ministry of Health, Social Services and Equality).
To assess and quantify the treatment costs, NSAIDs billing data in the SNS, in the year 2006 (group ATC M01A) compiled by the Ministry of Health, Social Services and Equality have been used. The use of other drugs has been assumed to be similar in the compared alternatives. For each one of the NSAID active substances included in the analysis, the RRP-VAT of the DDD billed with the different drug formulations has been considered. For ibuprofen, the formulations for paediatric use have been excluded from the analysis. Since the use of piroxicam has been strongly distorted by the regulatory measure, the year 2006, the year prior to the intervention, has been selected with the purpose of obtaining cost data (Table 3). The DDD costs of the analysed active substances were stable during the period 2006–2008, without changes in their reference prices. The average treatment cost has been obtained by multiplying the average cost of the respective DDD during the 20 considered days of treatment.
Treatment Cost Estimate With Non-steroidal Anti-inflammatory Drugs.
| Active substance | No. billed DDD | Amount at RRP-VAT | Cost per DDD |
|---|---|---|---|
| Piroxicam | 30 077 073 | 9 445 978.00€ | 0.31€ |
| Ibuprofen | 247 879 823 | 82 143 164.00€ | 0.33€ |
| Diclofenac | 129 015 044 | 32 991 315.00€ | 0.26€ |
| Naproxen | 80 164 895 | 14 150 173.00€ | 0.18€ |
| Aceclofenac | 40 829 701 | 25 670 667.00€ | 0.63€ |
DDD: defined daily dose.
Source: billing data in prescriptions to the National Healthcare System in the year 2006.
A time horizon of 1 year has been used.
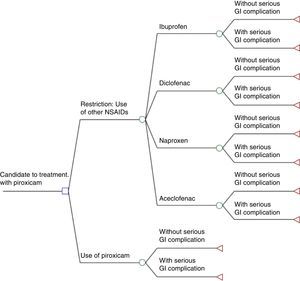
Cost-effectiveness Analysis and Decision-analytic ModelCosts (serious gastrointestinal complications diagnosis and hospital treatment and NSAIDs costs) and clinical effectiveness (serious gastrointestinal complications prevented) between the two alternatives have been assessed and compared: the intervention (actual) that took place in the year 2007 and implied a restriction on the use of piroxicam, and the assumption under which the aforesaid measure would have not been taken and piroxicam would have continued to be used without changes. To this end, a decision-analytic model has been used (decision tree), using the TreeAge Pro v11 program represented in Fig. 1.
Decision-analytic model. Initial medical problem: candidate to treatment with piroxicam through prescription in the National Health System with daily doses equal to one defined daily dose (DDD)×20 days, excluding people for whom prescription would still be possible after intervention. □ Decision node: the physician prescribes piroxicam (option that is only possible without the regulatory intervention) or, in the alternative situation with regulatory decision, the physician will no longer be able to prescribe piroxicam and will have to decide between ibuprofen, diclofenac, naproxen or aceclofenac. ○ Probability node: for the first option, there is a node with the probabilities of prescribing one of the non-steroidal anti-inflammatory drugs (NSAIDs) depending on the proportion of use in DDD of the 4 NSAIDs obtained from the billing data in prescription in the SNS in 2008. ○ Probability node (for all the previous decisions): the patient presents/does not present a serious gastrointestinal complication. The probabilities depend on the risk of serious gastrointestinal complications estimated for each NSAID.
We have performed the following univariate sensitivity analyses:
- (1)
Assumption of a higher likelihood of a patient suffering from a serious gastrointestinal complication, referring to studies which have estimated higher baseline incidences and relative risks than in the base case, and also applying the assumption of a longer average NSAID treatment duration:
- –
Baseline incidence obtained from the placebo groups in clinical trials,12 which is higher than the one estimated for the base case, which proceeds from observational studies in the general population.
- –
Relative risks of serious gastrointestinal complication for each NSAID: among the studies offering RR estimates for all implicated NSAIDs (Table 1), we have chosen the multicentre, observational study in Spanish hospitals,19 that obtained higher relative risks than those resulting from meta-analyses of all epidemiological studies.
- –
An average NSAID treatment duration of 60 days instead of 20 days.
- –
- (2)
Higher proportion of the use of ibuprofen and aceclofenac, which cost more than diclofenac and naproxen, in relation to the base case: 5% more use of each one of the two NSAIDs with higher DDD cost (ibuprofen and aceclofenac) and a decrease of 5% of each one of the two NSAIDs (diclofenac and naproxen) with lower DDD cost (Table 3).
- (3)
Average cost of a serious gastrointestinal complication with a minimum value equal to the lowest cost estimated in published studies, and a maximum value equal to the highest cost estimated in published studies. We have taken into account analyses published in the year 2000 that offer estimates with counts of the actual cost of serious gastrointestinal complication in public hospitals in Spain, updating the costs with the corresponding interannual inflation rates for each year after 2008 in which the data were gathered (Table 2).
A budget impact analysis was performed under the same assumptions used for the cost-effectiveness analysis. The average cost difference per every patient under treatment with piroxicam with respect to the intervention (use of one of the four NSAIDs) obtained in the cost-effectiveness analysis has been used to calculate the budget impact in Spain, which implied the replacement of piroxicam with less gastric-damaging NSAIDs during the first post-intervention year (2008).
To that effect, using the consumption data in SNS prescriptions, the population exposed to piroxicam during the year immediately before the intervention (year 2006) has been estimated, as well as the restricted population that continued using piroxicam in the year 2008, first complete year after piroxicam was transferred to hospital diagnosis as post-intervention scenario. As in the model used for the cost-effectiveness analysis, it was assumed that, as a consequence of the intervention, the population of patients that would have used piroxicam receives, instead, another NSAID (excluding those who continue taking piroxicam), and that this NSAID will be one of the four that are used the most in Spain.
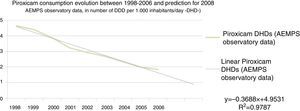
With the purpose of predicting the number of treatments with piroxicam in the year 2008 in a scenario in which the regulatory intervention would have not taken place in 2007, a regression line was performed with the consumption data of piroxicam in DDD per 1000 inhabitants/day (DHD) from the year 1998 until 2006, with the purpose of extrapolating the data to 2008 (Fig. 2). Thus, the use of piroxicam estimated for 2008 in a scenario in which the intervention would have not been passed would be of 0.8963 DDD per 1000 inhabitants/day (DHD).
ResultsIn Table 4, the data used as base for the calculation of health results and the costs of the analysed alternatives are summarised.
Summary of Data Used for Result Calculation in Health and Medical Costs. Base Case and Sensitivity Analysis.
| Variable | Source | Data | Base case | Sensitivity analysis |
|---|---|---|---|---|
| Proportion of use of each NSAID | % calculated over the sum of consumptions of the four NSAIDs in 2008 in no. of DDD billed in the SNS | % of ibuprofen (396 724 780 DDD) | 61.54% | (+5%) 66.54% |
| % of diclofenac (127 528 970 DDD) | 19.78% | (−5%) 14.78% | ||
| % of naproxen (84 310 276 DDD) | 13.08% | (−5%) 8.08% | ||
| % of aceclofenac (36 058 670 DDD) | 5.59% | (+5%) 10.59% | ||
| Baseline IR of serious gastrointestinal complications | Base case: Hernández-Diaz and Rodríguez (2002)16Sensitivity analysis: CNT collaboration (2013)20 | Baseline IR per 100 000 persons/year | 100 | 190 |
| Probability (IAtrto) of serious gastrointestinal complications (Table 1) | RR: base case: Castellsague et al. (2012)21Sensitivity analysis: Lanas et al. (2006)19IR in exposed: baseline IR×RRIAtrto=1−Exp (−IR×days of treatment) | Piroxicam IAtrto | 0.000407 | 0.003928 |
| Ibuprofen IAtrto | 0.000101 | 0.001280 | ||
| Diclofenac IAtrto | 0.000183 | 0.000968 | ||
| Naproxen IAtrto | 0.000225 | 0.002277 | ||
| Aceclofenac IAtrto | 0.000078 | 0.000968 | ||
| Medication cost: patient treated with NSAID (Table 2) | DDD cost: billing data in prescriptions to the SNS in 2006Base case: DDD cost×20 daysSensitivity analysis:DDD cost×60 days | Piroxicam cost | 6.28€ | 18.84€ |
| Ibuprofen cost | 6.63€ | 19.88€ | ||
| Diclofenac cost | 5.11€ | 15.34€ | ||
| Naproxen cost | 3.53€ | 10.59€ | ||
| Aceclofenac cost | 12.57€ | 37.72€ | ||
| Cost of disease: serious gastrointestinal complications (Table 3) | Base case: state rule AP-GRD V23Sensitivity analysis:Min: Rubio-Terrés (2010)11Max: Segú et al. (2002)7 | Cost in Euros of a serious gastrointestinal complication in the SNS in 2008 | 2876.39€ | 1944.85€3962.59€ |
NSAID: non-steroidal anti-inflammatory drug; DDD: defined daily dose; SGIC: serious gastrointestinal complications; DRG: diagnosis-related group; IAtrto: accumulated incidence associated to a patient under treatment with NSAID; RR: relative risk; SNS: National Healthcare System; IR: incidence rate in persons exposed/not exposed to NSAIDs.
In the base case, the average cost per patient for the intervention option (use of alternative NSAIDs to piroxicam) is of 6.64Euros, versus 7.45Euros per patient if the use of piroxicam had continued. This implies savings of 0.81Euros per patient. Regarding the effectiveness, the model foresees 1.32 cases of serious gastrointestinal complications per every 10,000 treated patients for a scenario in which patients are treated with alternative NSAIDs, and 4.07 cases for piroxicam (2.75 cases of serious gastrointestinal complications prevented per every 10 000 patients in a 20-day treatment). Therefore, the regulatory intervention happens to be the dominant option (Table 5).
Results of Cost-effectiveness Analysis and Impact of Restriction on the Use of Piroxicam in the First year Post-intervention.
| Costs (per patient in Euros) | Effectiveness (likelihood of suffering from a SGIC during treatment) | Budget impact | No. of cases of SGIC prevented in the population | |
|---|---|---|---|---|
| Intervention (use of alternative NSAIDs to piroxicam) | 6.64€ | 1.32/10 000 | ||
| No intervention (use of piroxicam) | 7.45€ | 4.07/10 000 | ||
| Base case result (difference) | 0.81€ of saving per patient | 2.75/10 000 cases prevented per 10 000 treatments | −578 608€ | 196 |
| Sensitivity analysis results | ||||
| 1. Risks of major SGIC | −7.50€ | 26.00/10 000 | −1 785 827€ | 619 |
| 2. Higher use of alternative NSAIDs with higher treatment cost | −0.32€ | 2.86/10 000 | −228 586€ | 205 |
| 3. Cost variability of SGIC, see Table 2 (minimum–maximum) | −0.56€ to −1.11€ | 2.75/10 000 | −400 025€ to −792 907€ | 196 |
NSAID: non-steroidal anti-inflammatory drug; SGIC: serious gastrointestinal complications.
The univariate sensitivity analysis also offers a dominant result that favours the regulatory intervention option for the three analysed variables. The base case continues to be the most conservative option in regards to effectiveness, and with the scenario of higher risks of serious gastrointestinal complications, the saving per patient reaches 7.50Euros and 26 cases of serious gastrointestinal complications prevented per every 10,000 patients under treatment.
Budget Impact and Serious Gastrointestinal Complications PreventedWe gathered that in Spain, in the year 2008, assuming the replacement of piroxicam with one of the four reference NSAIDs, the saving in 1 year for the SNS is estimated in −578 608 Euros (Table 6). After performing a sensitivity analysis using the same variables used for the cost-effectiveness analysis, the saving ranges between 228 586 Euros for the case in which as a replacement for piroxicam, the most expensive NSAIDs were used in a higher percentage (10% more), and 1 785 827 Euros for the case in which the risks of serious gastrointestinal complications were higher than those used in the base case.
Sources of Information in the Calculations for Budget Impact Analysis.
| Data and source of information | Scenario with intervention (restricted use of piroxicam) | Scenario without intervention (use of piroxicam) |
|---|---|---|
| No. Total DDD | 813 892 DDD(prescription billing year 2008) | 15 100 508 DDD(extrapolation from previous years) |
| No. DDD target population of the intervention | 14 286 616 DDD | |
| Target population of patients under treatment(20-day treatments with daily dose equal to DDD) | 714 331 patients | |
| Cost variance per patient(Source: study cost-effectiveness analysis) | −0.81 Euros | |
| Budget impact | −578 608 Euros |
DDD: defined daily dose.
The study shows that interventions in pharmacovigilance do not only result beneficial for public health by limiting the risk of adverse reactions to drugs (around 200 cases of serious gastrointestinal complications prevented in 1 year,) but they may also have a favourable financial impact on the health care system (near 600,000 Euros saved to the SNS in 2008).
The study poses a somehow innovative methodological approach. On the one hand, it follows a similar methodology to a complete economic assessment performed when a new drug or therapeutic strategy is introduced. In other words, the intervention costs and consequences are analysed in comparison with those of the non-intervention scenario. However, in this case, the intervention consists in restricting the use of a drug, instead of introducing it into the market, and the consequences are those derived from the prevention of the health problem by replacing the problematic drug with safer alternatives. Moreover, the sources of information are all retrospective since the intervention has already taken place, so we need less assumptions and modelling than when the analyses must anticipate a future scenario.
Another aspect to be taken into account is that the analysis performed may be considered conservative in different aspects. Thus, the time horizon has been limited to 1 year. Beyond that period of time, it carries more uncertainties to assume that other actions different from the regulatory measure may have affected the use of piroxicam. In fact, it is observed that the use of piroxicam in Spain was already falling in the years prior to the regulatory measure (Fig. 2), which has been taken into account in the analysis. It is clear that this descent was influenced by a higher awareness among prescribers regarding this drug's gastrointestinal risks, as well as the recommendations, from several instances, favouring the use of NSAIDs such as ibuprofen, diclofenac or naproxen, with a lower gastrointestinal risk, as shown by the data presented.
As far as the source of information regarding health results is concerned, i.e., the risk of serious gastrointestinal complications with the different NSAIDs, data from observational studies have been used. In this case, the possible alternative of using data from clinical trials is, on the one hand, not feasible, since there are no reliable data from clinical trials on the risk of serious gastrointestinal complications with piroxicam. And, on the other hand, in this scenario, data from observational studies, obtained from a population with demographic characteristics and risk factors more representative of the general population, offer more reliable and representative information, as already stated by other authors.24 Moreover, in the interest of performing a more conservative analysis, sources of information have been selected in the base case, about the risk of serious gastrointestinal complications that estimate the lowest risks for piroxicam, in comparison with, for example, those of the study used in the sensitivity analysis,19 which is also representative of the hospital admissions for serious gastrointestinal complications in Spain. On the other hand, the average treatment duration selected in the base case (20 days) is much shorter than the one used, for example, in the models of pharmacoeconomic studies performed when Coxibs were placed into market, where model scenarios were based on clinical trials in chronic rheumatic pathology and treatment duration of 6 months to 1 year, even higher than the 60 days used in the sensitivity analysis for this study.
As a limitation to the study, we have assumed that there are no differences in other risks different from the serious gastrointestinal complications between the analysed drugs. For cardiovascular risks, not considering these possible differences could be relevant. The NSAIDs’ cardiovascular risks have been thoroughly analysed in a recent review in Europe.25 However, no strong conclusions were reached for NSAIDs which, as piroxicam, have not been analysed in the major studies performed on this subject. In this sense, the most used NSAID, ibuprofen, shows no favourable or unfavourable difference compared with piroxicam, and data from a recent study in Spain26 indicate that it probably does not present advantages with respect to ibuprofen in this regard. On the other hand, it is important to stress out that current data show that aceclofenac and diclofenac would have a higher cardiovascular risk, while naproxen would have a relatively lower risk, compared to the usual doses of ibuprofen, and that these differences between the different substitute NSAIDs of piroxicam have not been considered in the model. In regards to other possible risks, some epidemiological studies have shown a higher risk in the oxicam group of NSAIDs (to which piroxicam belongs) of producing more serious skin reactions, though with a very low incidence. In any case, this would give piroxicam an additional disadvantage. Likewise, less serious gastrointestinal adverse effects have not been taken into account, such as non-complicated ulcers or general digestive intolerance, which could be argued to be more common with piroxicam, but with incidence data, compared to other less solid NSAIDs.
Another limitation of the study is the use of aggregate data on drug use in the SNS and on DDD to estimate the profile of use of NSAIDs in Spain. Therefore, estimates of population under treatment assume an average treatment duration and an average treatment dose, while in fact there could be a significant variability that is not taken into account in the analyses performed. In regards to the DDD, even though its use allows for comparisons in the use of medications in time and among different places, it does not have to represent the actual dose taken by patients. In regards to the NSAIDs, data from actual prescriptions in Spain13 indicate that the naproxen DDD (0.5g) may be lower than the actual average daily dose. If this occurred differentially with naproxen, the consequences in the model results would be, in any case, very limited. We would have incurred in an underestimation of cost with this NSAID but also in an overestimation of its prevalence of use, which would also imply more cases of SGIC prevented since the gastrointestinal risk of naproxen is slightly higher than in the other NSAIDs from the piroxicam substitute's model (Table 1).
In this sense, the performance of future studies with a similar approach, but resorting to sources of information on the use of drugs with the patients’ individual data, such as the BIFAP (Base de datos para la Investigación Farmacoepidemiológica en Atención Primaria [Pharmaco-Epidemiological Research in Primary Care]) database,27 would allow to settle these issues and enable the assessment of the consequences of qualitative changes in the patterns of use of drugs. Besides, this type of database would enable the performance of simulations to see the consequences of the regulatory measures, including the financial consequences.
Finally, this study shows that it is possible to perform complete economic assessments on pharmacovigilance activities. The intervention performed in 2007 by AEMPS on piroxicam not only fulfilled its purpose of preventing adverse reactions, but also implied a significant saving, even under conservative assumptions. In conclusion, the determination of the scope of the economic benefits produced by this or other decisions in pharmacovigilance allows for the contribution of new arguments to invest in these public health activities.
Ethical ResponsibilitiesProtection of persons and animalsAuthors state that no experiments were performed on human beings or animals as part of this investigation.
Data confidentialityAuthors state that this article does not contain patient data.
Right to privacy and informed consentAuthors state that this article does not contain patient data.
FundingNone.
Conflict of InterestsNone.
To professor Félix Lobo Aleu, director of the Masters in Health Assessment and Market Access (Pharmaco-Economy) course of study of the Universidad Carlos III in Madrid, and under whose direction this study was presented as Master's degree final project.
Please cite this article as: Maciá Martínez M-Á. Evaluación económica de la restricción del uso de piroxicam en España. Reumatol Clin. 2015;11:345–352.