Due to the clinical heterogeneity of psoriatic arthritis (PsA), recommendations have been developed by international groups to guide therapeutic decisions of the rheumatologist. The objective of the current systematic review (RS) was to evaluate the evidence of efficacy of disease-modifying antirheumatic drugs (DMARDs) in PsA.
MethodsLiterature search in Medline, EMBASE, Cochrane Library, from 2008 to 2014. We included RS, randomized clinical trials and observational studies, in patients with PsA and an evaluation of efficiency of conventional DMARDs (methotrexate, sulfasalazine, leflunomide), according to the following outcomes: peripheral and axial symptoms; peripheral radiological damage; enthesitis according to power Doppler ultrasound or magnetic resonance imaging (enthesitis count before and after therapy); dactylitis; uveitis.
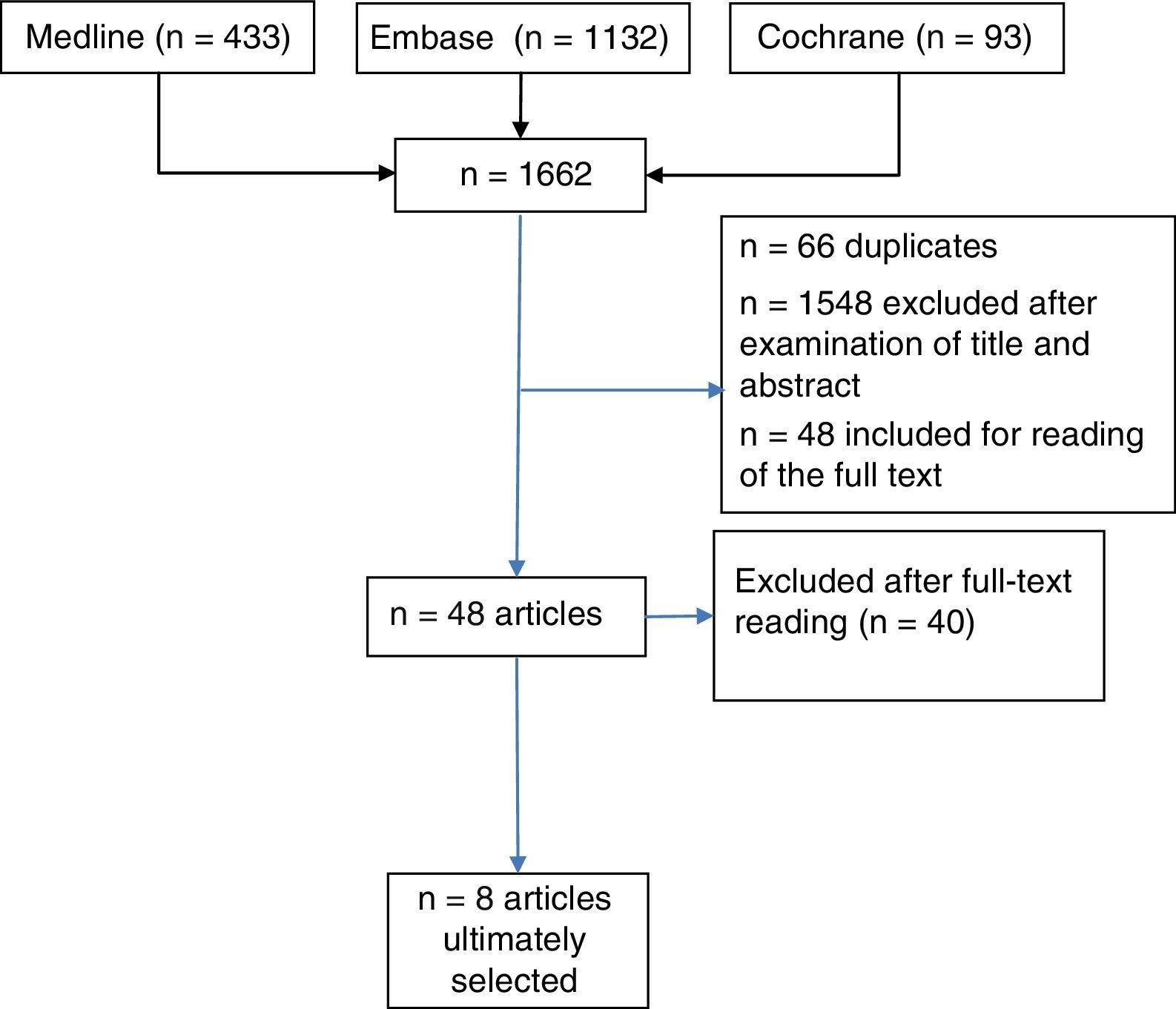
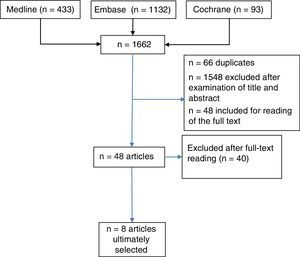
ResultsTitle and abstract were used to retrieve 1662 documents for this review (Medline, n=433; EMBASE n=1132; Cochrane, n=97), and 48 studies were selected for detailed reading; finally, 8 studies were included.
ConclusionsSince the studies included are not robust, and there are arguments to support the effectiveness of methotrexate, the evidence observed with the treatment of DMARDs in PsA is not conclusive.
Dada la heterogeneidad clínica de la artritis psoriásica (APs), se han elaborado recomendaciones por grupos internacionales para orientar las decisiones terapéuticas del reumatólogo. Esta revisión sistemática (RS) tiene el objetivo de evaluar la evidencia sobre la eficacia de los FAME en APs.
MétodosBúsqueda bibliográfica en Medline, Embase, Cochrane Library, desde 2008 hasta 2014. Se incluyeron RS, EC y estudios observacionales, en pacientes con APs con evaluación de eficacia de FAME sintéticos (metotrexato, sulfasalazina y leflunomida), los siguientes desenlaces: síntomas periféricos; daño estructural radiológico periférico; síntomas axiales; entesopatía por ecografía o resonancia magnética (número de entesis antes y después del estudio); dactilitis, y uveítis.
ResultadosSe recuperaron 1.662 documentos para revisar por título y «abstract» (Medline, n=433; Embase n=1.132; Cochrane, n=97), se seleccionaron 48 estudios para su lectura detallada, y se incluyeron 8 estudios.
ConclusionesYa que los estudios incluidos no son consistentes, y hay argumentos para apoyar la eficacia del metotrexato, la evidencia observada con el tratamiento de FAME en APs no es concluyente.
Psoriatic arthritis (PsA) is a systemic inflammatory disease that affects 20%–30% of patients with psoriasis. It is characterized by inflammation involving the musculoskeletal system and the skin, including its clinical manifestations, the axial spine, peripheral joints, enthesitis, dactylitis and nail and skin lesions.1 Certain patients have a mild course, whereas others can develop radiological joint damage, peripheral joint destruction and functional disability. Systematic reviews (SR) have been carried out, some with a meta-analysis,2–4 on the efficacy of treatment in PsA,2–5 and have demonstrated a low level of evidence (LE) regarding the effectiveness of synthetic disease-modifying antirheumatic drugs (DMARDs). However, in the attempt to offer guidance to specialists, given the heterogeneity of the clinical presentation and the different degrees of severity in joint involvement,1 2 international groups, on the basis of those SR, have formulated recommendations concerning treatment. These groups are the European League Against Rheumatism (EULAR),6 with an algorithm focused mainly on musculoskeletal symptoms, and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA),7 that evaluated 5 domains (peripheral arthritis, skin and nail involvement, enthesitis, dactylitis and axial arthritis).8 As a result, for active peripheral arthritis, both recommended treatment with DMARDs, such as methotrexate (MTX), sulfasalazine (SSZ) and leflunomide (LEF).
Methotrexate has frequently been utilized as the first DMARD to be administered in PsA because of the efficacy it shows in the treatment of joint and skin disorders.9 Despite the low LE, MTX is still one of the most widely used drugs in PsA.1 Thirty percent of the visits to the rheumatology department of patients referred from dermatology involves the addition of a new DMARD, with MTX being that most frequently employed.10 The scarcity of high-quality clinical trials supporting its efficacy in PsA compelled us to ask the reason for the generalized use of MTX in PsA,1 its effectiveness and for which clinical phenotype of PsA. Moreover, although clinical trials have not shown that the efficacy of MTX versus placebo is sufficiently significant, it is considered that the design of those studies had methodological limitations that make it unviable to draw definitive conclusions.11 Therefore, the objective of this report was to carry out a systematic reassessment of the available evidence on the utility of DMARDs in the management of PsA. This forms part of the process of updating the clinical practice guidelines for the treatment of axial spondyloarthritis and PsA of the Spanish Society of Rheumatology (SER) (ESPOGUIA),12 the previous version of which was issued in 2009.
Material and MethodsWe performed a SR in 3 databases: Medline, EMBASE and the Cochrane Library. They included studies in English, Spanish and French, dating from January 2008 to November 2014, to retrieve articles on the efficacy of DMARDs in PsA. The terms employed for the search strategy involving high-sensitivity descriptors (MG) are shown in the Supplementary appendix.
Inclusion criteria: using the population, intervention, comparison and outcome (PICO) format, we selected those randomized controlled trials (RCT) that met the following requirements: (1) adults with PsA with a patient population of 50 or more individuals; (2) intervention with traditional DMARDs (MTX, SSZ, LEF) versus placebo; (3) efficacy outcome measures in terms of changes in: (a) peripheral symptoms (American College of Rheumatology [ACR] 20/50/70, EULAR response based on Disease Activity Score in 28 joints, Psoriatic Arthritis Response Criteria [PsARC]; peripheral radiological structural damage [Sharp/van der Heijde score—hands, wrists and feet—modified for PsA, which includes distal interphalangeal joints); (b) axial involvement (Bath Ankylosing Spondylitis Disease Activity Index, Bath Ankylosing Spondylitis Functional Index, Assessment of SpondyloArthritis international Society [ASAS] 20/40 5/6); (c) enthesopathy according to ultrasound or magnetic resonance imaging with number of entheses (Maastricht Ankylosing Spondylitis Enthesitis Score, Leeds Enthesitis Index) on inclusion and at the end of the study (percentage improvement); (d) dactylitis, with number of affected digits at baseline and at the end of the study (percentage improvement); and (e) uveitis, with number of episodes prior to and after treatment. We included SR of RCT (with preference for phase III or IV). We also selected studies that helped to partially respond to the question posed (efficacy and safety of a traditional DMARD versus a traditional DMARD or versus a combination of several traditional DMARDs). When there was no existing evidence on a question because of the design of a SR of RCT, we considered observational studies included in the SR. We excluded reports that did not adjust to the components of the PICO question, as well as abstracts, posters, narrative reviews, letters, editorials and any text that had not been published. The selection of the studies was performed by a reviewer (JM) in successive phases: selection of titles and abstracts from the selected titles, review of the complete text of the selected studies and their evaluation, to eliminate articles that did not meet the inclusion criteria. We also performed a hand-search in the bibliography of the studies included. Doubts that arose during the selection process were weighed by 2 methodologists from the SER (PD, DS) and a consensus was reached in every case. To manage the literature references we utilized EndNote X7. In the critical reading of the articles and the evaluation of the quality, we employed the checklists of the Scottish Intercollegiate Guidelines Network (SIGN).13 To assign the LE, we used the Oxford system.14
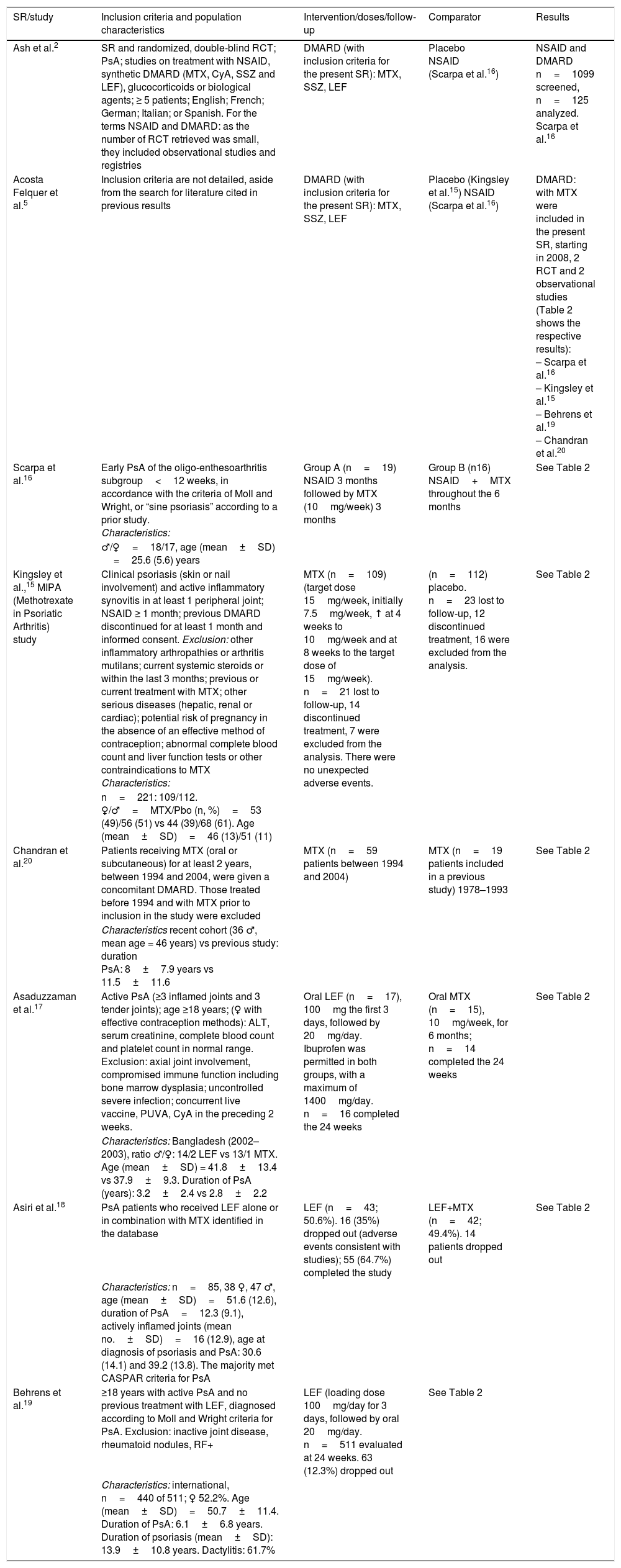
ResultsWe retrieved 1662 documents to review on the basis of their title and abstract (Medline, n=433; EMBASE, n=1132; Cochrane, n=97). In all, 48 articles were selected for full-text reading; of these, 40 were excluded (and can be made available on request). Ultimately, 8 studies met the inclusion criteria (Fig. 1) (Tables 1 and 2 providing evidence synthesis). In all, there were 2 SR,2,5 2 RCT included in 1 of these SR,15,16 an open-label RCT not included in the SR,17 3 observational studies (2 prospective studies18,19 and 1 retrospective20); 2 of the latter18,19 were reviewed in the SR of Acosta Felquer et al.,5 with a LE that is summarized in Table 3. Methotrexate was the drug considered in the intervention in 2 RCT15,16 and in an observational study,20 whereas LEF was the subject in 3 articles17–19: an open-label RCT17 and 2 observational studies.18,19
Systematic Review and Studies Included (Inclusion Criteria, Population Characteristics, Intervention, Follow-up).
| SR/study | Inclusion criteria and population characteristics | Intervention/doses/follow-up | Comparator | Results |
|---|---|---|---|---|
| Ash et al.2 | SR and randomized, double-blind RCT; PsA; studies on treatment with NSAID, synthetic DMARD (MTX, CyA, SSZ and LEF), glucocorticoids or biological agents; ≥ 5 patients; English; French; German; Italian; or Spanish. For the terms NSAID and DMARD: as the number of RCT retrieved was small, they included observational studies and registries | DMARD (with inclusion criteria for the present SR): MTX, SSZ, LEF | Placebo NSAID (Scarpa et al.16) | NSAID and DMARD n=1099 screened, n=125 analyzed. Scarpa et al.16 |
| Acosta Felquer et al.5 | Inclusion criteria are not detailed, aside from the search for literature cited in previous results | DMARD (with inclusion criteria for the present SR): MTX, SSZ, LEF | Placebo (Kingsley et al.15) NSAID (Scarpa et al.16) | DMARD: with MTX were included in the present SR, starting in 2008, 2 RCT and 2 observational studies (Table 2 shows the respective results): – Scarpa et al.16 – Kingsley et al.15 – Behrens et al.19 – Chandran et al.20 |
| Scarpa et al.16 | Early PsA of the oligo-enthesoarthritis subgroup<12 weeks, in accordance with the criteria of Moll and Wright, or “sine psoriasis” according to a prior study. Characteristics: | Group A (n=19) NSAID 3 months followed by MTX (10mg/week) 3 months | Group B (n16) NSAID+MTX throughout the 6 months | See Table 2 |
| ♂/♀=18/17, age (mean±SD) =25.6 (5.6) years | ||||
| Kingsley et al.,15 MIPA (Methotrexate in Psoriatic Arthritis) study | Clinical psoriasis (skin or nail involvement) and active inflammatory synovitis in at least 1 peripheral joint; NSAID ≥ 1 month; previous DMARD discontinued for at least 1 month and informed consent. Exclusion: other inflammatory arthropathies or arthritis mutilans; current systemic steroids or within the last 3 months; previous or current treatment with MTX; other serious diseases (hepatic, renal or cardiac); potential risk of pregnancy in the absence of an effective method of contraception; abnormal complete blood count and liver function tests or other contraindications to MTX Characteristics: | MTX (n=109) (target dose 15mg/week, initially 7.5mg/week, ↑ at 4 weeks to 10mg/week and at 8 weeks to the target dose of 15mg/week). n=21 lost to follow-up, 14 discontinued treatment, 7 were excluded from the analysis. There were no unexpected adverse events. | (n=112) placebo. n=23 lost to follow-up, 12 discontinued treatment, 16 were excluded from the analysis. | See Table 2 |
| n=221: 109/112. ♀/♂=MTX/Pbo (n, %)=53 (49)/56 (51) vs 44 (39)/68 (61). Age (mean±SD)=46 (13)/51 (11) | ||||
| Chandran et al.20 | Patients receiving MTX (oral or subcutaneous) for at least 2 years, between 1994 and 2004, were given a concomitant DMARD. Those treated before 1994 and with MTX prior to inclusion in the study were excluded | MTX (n=59 patients between 1994 and 2004) | MTX (n=19 patients included in a previous study) 1978–1993 | See Table 2 |
| Characteristics recent cohort (36 ♂, mean age = 46 years) vs previous study: duration PsA: 8±7.9 years vs 11.5±11.6 | ||||
| Asaduzzaman et al.17 | Active PsA (≥3 inflamed joints and 3 tender joints); age ≥18 years; (♀ with effective contraception methods): ALT, serum creatinine, complete blood count and platelet count in normal range. Exclusion: axial joint involvement, compromised immune function including bone marrow dysplasia; uncontrolled severe infection; concurrent live vaccine, PUVA, CyA in the preceding 2 weeks. | Oral LEF (n=17), 100mg the first 3 days, followed by 20mg/day. Ibuprofen was permitted in both groups, with a maximum of 1400mg/day. n=16 completed the 24 weeks | Oral MTX (n=15), 10mg/week, for 6 months; n=14 completed the 24 weeks | See Table 2 |
| Characteristics: Bangladesh (2002–2003), ratio ♂/♀: 14/2 LEF vs 13/1 MTX. Age (mean±SD) = 41.8±13.4 vs 37.9±9.3. Duration of PsA (years): 3.2±2.4 vs 2.8±2.2 | ||||
| Asiri et al.18 | PsA patients who received LEF alone or in combination with MTX identified in the database | LEF (n=43; 50.6%). 16 (35%) dropped out (adverse events consistent with studies); 55 (64.7%) completed the study | LEF+MTX (n=42; 49.4%). 14 patients dropped out | See Table 2 |
| Characteristics: n=85, 38 ♀, 47 ♂, age (mean±SD)=51.6 (12.6), duration of PsA=12.3 (9.1), actively inflamed joints (mean no.±SD)=16 (12.9), age at diagnosis of psoriasis and PsA: 30.6 (14.1) and 39.2 (13.8). The majority met CASPAR criteria for PsA | ||||
| Behrens et al.19 | ≥18 years with active PsA and no previous treatment with LEF, diagnosed according to Moll and Wright criteria for PsA. Exclusion: inactive joint disease, rheumatoid nodules, RF+ | LEF (loading dose 100mg/day for 3 days, followed by oral 20mg/day. n=511 evaluated at 24 weeks. 63 (12.3%) dropped out | See Table 2 | |
| Characteristics: international, n=440 of 511; ♀ 52.2%. Age (mean±SD)=50.7±11.4. Duration of PsA: 6.1±6.8 years. Duration of psoriasis (mean±SD): 13.9±10.8 years. Dactylitis: 61.7% |
ALT, alanine transaminase; CyA, cyclosporine; DMARD, disease-modifying antirheumatic drug; LEF, leflunomide; MTX, methotrexate; NSAID, nonsteroidal anti-inflammatory drug; PsA, psoriatic arthritis; RCT, randomized controlled trial; PGA, pain (patient global assessment; PhGA, physician global assessment; Pbo, placebo; PUVA, psoralen ultraviolet A; RF, rheumatoid factor; SD, standard deviation; SR, systematic review; SSZ, sulfasalazine; TJC, tender joint count.
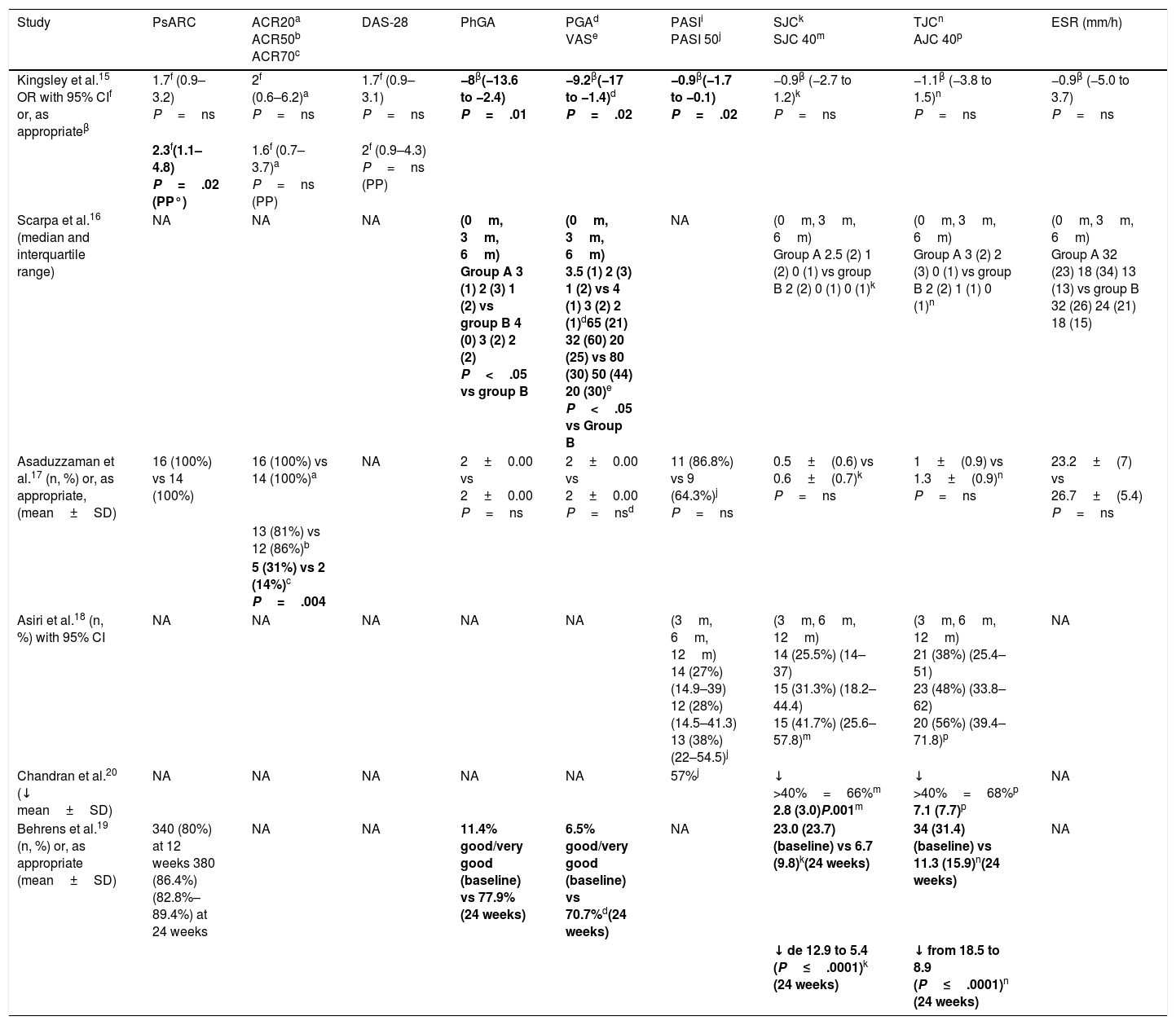
Results of the Studies Included.
| Study | PsARC | ACR20a ACR50b ACR70c | DAS-28 | PhGA | PGAd VASe | PASIi PASI 50j | SJCk SJC 40m | TJCn AJC 40p | ESR (mm/h) |
|---|---|---|---|---|---|---|---|---|---|
| Kingsley et al.15 OR with 95% CIf or, as appropriateβ | 1.7f (0.9–3.2) P=ns | 2f (0.6–6.2)a P=ns | 1.7f (0.9–3.1) P=ns | −8β(−13.6 to −2.4) P=.01 | −9.2β(−17 to −1.4)d P=.02 | −0.9β(−1.7 to −0.1) P=.02 | −0.9β (−2.7 to 1.2)k P=ns | −1.1β (−3.8 to 1.5)n P=ns | −0.9β (−5.0 to 3.7) P=ns |
| 2.3f(1.1–4.8) P=.02 (PP°) | 1.6f (0.7–3.7)a P=ns (PP) | 2f (0.9–4.3) P=ns (PP) | |||||||
| Scarpa et al.16 (median and interquartile range) | NA | NA | NA | (0m, 3m, 6m) Group A 3 (1) 2 (3) 1 (2) vs group B 4 (0) 3 (2) 2 (2) P<.05 vs group B | (0m, 3m, 6m) 3.5 (1) 2 (3) 1 (2) vs 4 (1) 3 (2) 2 (1)d65 (21) 32 (60) 20 (25) vs 80 (30) 50 (44) 20 (30)e P<.05 vs Group B | NA | (0m, 3m, 6m) Group A 2.5 (2) 1 (2) 0 (1) vs group B 2 (2) 0 (1) 0 (1)k | (0m, 3m, 6m) Group A 3 (2) 2 (3) 0 (1) vs group B 2 (2) 1 (1) 0 (1)n | (0m, 3m, 6m) Group A 32 (23) 18 (34) 13 (13) vs group B 32 (26) 24 (21) 18 (15) |
| Asaduzzaman et al.17 (n, %) or, as appropriate, (mean±SD) | 16 (100%) vs 14 (100%) | 16 (100%) vs 14 (100%)a | NA | 2±0.00 vs 2±0.00 P=ns | 2±0.00 vs 2±0.00 P=nsd | 11 (86.8%) vs 9 (64.3%)j P=ns | 0.5±(0.6) vs 0.6±(0.7)k P=ns | 1±(0.9) vs 1.3±(0.9)n P=ns | 23.2±(7) vs 26.7±(5.4) P=ns |
| 13 (81%) vs 12 (86%)b | |||||||||
| 5 (31%) vs 2 (14%)c P=.004 | |||||||||
| Asiri et al.18 (n, %) with 95% CI | NA | NA | NA | NA | NA | (3m, 6m, 12m) 14 (27%) (14.9–39) 12 (28%) (14.5–41.3) 13 (38%)(22–54.5)j | (3m, 6m, 12m) 14 (25.5%) (14–37) 15 (31.3%) (18.2–44.4) 15 (41.7%) (25.6–57.8)m | (3m, 6m, 12m) 21 (38%) (25.4–51) 23 (48%) (33.8–62) 20 (56%) (39.4–71.8)p | NA |
| Chandran et al.20 (↓ mean±SD) | NA | NA | NA | NA | NA | 57%j | ↓ >40%=66%m 2.8 (3.0)P.001m | ↓ >40%=68%p 7.1 (7.7)p | NA |
| Behrens et al.19 (n, %) or, as appropriate (mean±SD) | 340 (80%) at 12 weeks 380 (86.4%) (82.8%–89.4%) at 24 weeks | NA | NA | 11.4% good/very good (baseline) vs 77.9% (24 weeks) | 6.5% good/very good (baseline) vs 70.7%d(24 weeks) | NA | 23.0 (23.7) (baseline) vs 6.7 (9.8)k(24 weeks) | 34 (31.4) (baseline) vs 11.3 (15.9)n(24 weeks) | NA |
| ↓ de 12.9 to 5.4 (P≤.0001)k (24 weeks) | ↓ from 18.5 to 8.9 (P≤.0001)n (24 weeks) |
The cells in boldface are those in which the P value is significant.
ACR, American College of Rheumatology; ACR50, American College of Rheumatology criterion indicating an improvement of at least 50%; ACR20 indicates an improvement of ≥20%; ACR70 indicates an improvement of ≥70%; AJC 40, indicates a decrease in more than 40% of the actively inflamed joints; CI, confidence interval; DAS, disease activity score; ESR, erythrocyte sedimentation rate; m, month; NA, not applicable; ns, P value not significant; OR, odds ratio; PASI, Psoriasis Area and Severity Index; PGA, patient global assessment; PhGA, physician global assessment; PP, protocol analysis; PsARC, Psoriatic Arthritis Response Criteria; SD, standard deviation; SJC, swollen joint count; SJC 40, decrease in swollen joints by more than 40%; VAS, Visual Analog Scale.
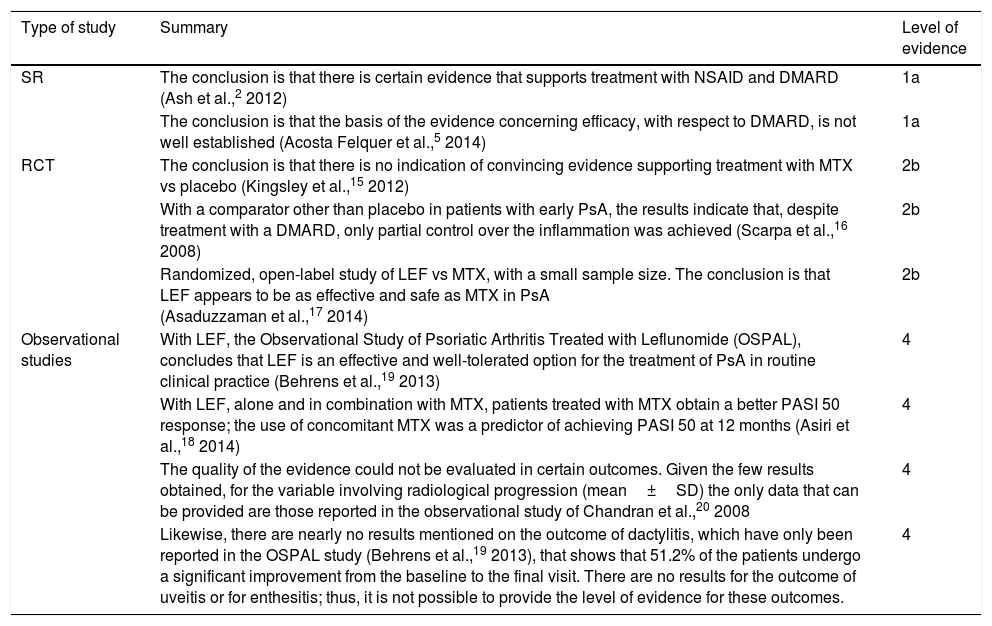
Summary of the Evidence: Level and Quality of Evidence.
| Type of study | Summary | Level of evidence |
|---|---|---|
| SR | The conclusion is that there is certain evidence that supports treatment with NSAID and DMARD (Ash et al.,2 2012) | 1a |
| The conclusion is that the basis of the evidence concerning efficacy, with respect to DMARD, is not well established (Acosta Felquer et al.,5 2014) | 1a | |
| RCT | The conclusion is that there is no indication of convincing evidence supporting treatment with MTX vs placebo (Kingsley et al.,15 2012) | 2b |
| With a comparator other than placebo in patients with early PsA, the results indicate that, despite treatment with a DMARD, only partial control over the inflammation was achieved (Scarpa et al.,16 2008) | 2b | |
| Randomized, open-label study of LEF vs MTX, with a small sample size. The conclusion is that LEF appears to be as effective and safe as MTX in PsA (Asaduzzaman et al.,17 2014) | 2b | |
| Observational studies | With LEF, the Observational Study of Psoriatic Arthritis Treated with Leflunomide (OSPAL), concludes that LEF is an effective and well-tolerated option for the treatment of PsA in routine clinical practice (Behrens et al.,19 2013) | 4 |
| With LEF, alone and in combination with MTX, patients treated with MTX obtain a better PASI 50 response; the use of concomitant MTX was a predictor of achieving PASI 50 at 12 months (Asiri et al.,18 2014) | 4 | |
| The quality of the evidence could not be evaluated in certain outcomes. Given the few results obtained, for the variable involving radiological progression (mean±SD) the only data that can be provided are those reported in the observational study of Chandran et al.,20 2008 | 4 | |
| Likewise, there are nearly no results mentioned on the outcome of dactylitis, which have only been reported in the OSPAL study (Behrens et al.,19 2013), that shows that 51.2% of the patients undergo a significant improvement from the baseline to the final visit. There are no results for the outcome of uveitis or for enthesitis; thus, it is not possible to provide the level of evidence for these outcomes. | 4 |
DMARD, disease-modifying antirheumatic drug; LEF, leflunomide; MTX, methotrexate; NSAID, nonsteroidal anti-inflammatory drug; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; RCT, randomized controlled trial; SD, standard deviation; SR, systematic review.
The SR published by Ash et al.2 was carried out in 2012, with studies conducted between 1962 and January 2010. Their objective was to review the available evidence on the efficacy and safety of all the non-topical drug therapies for PsA, from nonsteroidal anti-inflammatory drugs (NSAID) to DMARDs and biological agents, to provide data on the development of EULAR recommendations for the management of PsA (LE 1a). Likewise, in the SR by Acosta Felquer et al.,5 in 2014, the objective was to evaluate the efficacy of different treatments and therapeutic strategies to provide information for the development of new GRAPPA recommendations for the treatment of peripheral PsA, and summarize the evidence on the available therapies. They searched for documentation in Medline, EMBASE and the Cochrane Library, from 2006 to the present. They also reviewed abstracts from the EULAR and the ACR, from 2010 to 2013. In the SR by Ash et al., the authors concluded that there was some evidence to support treatment with NSAIDs and DMARDs, with good evidence of the efficacy of anti-tumor necrosis factor agents in PsA,2 whereas, with respect to DMARDs, the conclusions of the SR of Acosta Felquer et al. were that the basis for the evidence of their effectiveness was not well established.5
In the analysis of the studies retrieved for the SR by Ash et al.,2 with regard to the use of MTX, there were 3 RCT (n=93 patients) that compared MTX in monotherapy vs placebo and 7 open-label or retrospective studies on MTX in PsA. These studies did not meet the inclusion criteria for the present SR. Likewise, when the intervention involved the administration of LEF, several of the studies included did not comply with the inclusion criteria for the present SR, among them a RCT and 2 open-label trials. When the drug employed was SSZ, the reports cited to evaluate its efficacy (7 RCT that compared SSZ in monotherapy vs placebo or symptomatic treatment) corresponded to evidence prior to 2008 and included in the 2009 ESPOGUIA.
MethotrexateThe open-label RCT by Scarpa et al.,16 cited in both SR,2,5 evaluates the efficacy of MTX against a comparator other than placebo (NSAID), at 3 and 6 months, in a group of patients with early PsA (n=35), who were divided into 2 groups according to the type of joint involvement (Table 1). The group that took MTX from the beginning achieved improvement more rapidly in terms of the tender joint count (TJC) and swollen joint count (SJC), a difference that was statistically significant at 3 months (P<.05). At 6 months, all of the variables in the 2 groups confirmed the previously recorded improvement, but, in the comparison of the groups, except for a significant improvement in the patient global assessment (PGA) and physician global assessment (PhGA) in the group that added MTX at 3 months, there were no statistically significant results (P<.05) (Table 2). According to these findings, it was concluded that MTX in early PsA is effective against the clinical symptoms, whereas the majority of the markers of disease activity were not substantially affected. In that open-label RCT, we observe that the sample size was small and the study was limited to early PsA, without mentioning the number of dropouts (Table 3) (LE 2b).
The RCT by Kingsley et al., a study from the Methotrexate in Psoriatic Arthritis (MIPA) trial,15 was conducted for the purpose of confirming the efficacy of MTX (15mg/week). It had a randomized, placebo-controlled, double-blind, parallel-group design and was performed in patients with active PsA. The primary outcome measure was PsARC at 6 months and other outcome measures included were ACR20, DAS28 and their components. The authors randomized 221 patients, n=109 with MTX and n=112 with placebo, and analyzed 67 (61%) who completed the study and 61 (54%) in the active and placebo groups, respectively (Table 1). The only benefits of MTX were the lower scores in the patient and physician global assessments (PGA/PhGA) and the mean score for skin involvement (Psoriasis Area and Severity Index [PASI]) at 6 months (Table 2). They concluded that there was no evidence indicating an improvement in synovitis with MTX and, consequently, question its classification as a DMARD in PsA. However, there are factors in the study design that could contribute to the results observed, including a large number of individuals who were lost to follow-up, which reduces the validity and the conclusion of the study, which could reflect attrition bias and, thus, bias the results. It took 5 years to recruit the participants, a fact that could reflect an element of selection bias. Around 35% of the patients included had oligoarticular disease and a relatively low MTX dose, a finding recorded in only 78% of the patients (Table 3) (LE 2b).
In the retrospective observational study by Chandran et al.,20 the primary objective was to compare their findings with those of a previous study to determine whether starting treatment with MTX earlier in the course of PsA, and using a higher dose, led to better outcomes in the progression of radiological peripheral joint damage (modified Steinbrocker method) (Table 1). As in their previous study, the authors employed as an outcome measure a reduction ≥40% in actively inflamed joints (active joint count [ACJ] 40) and the PASI for psoriasis. With a shorter duration of PsA in the cohort described in this report (mean±standard deviation [SD]) of 8±7.9 vs 11.5±11.6 years and a higher dose of MTX (16.2 vs 10.8mg/week), the outcome (mean±SD) showed significantly less radiological progression in the more recent cohort (1.5±1.8) with respect to the earlier patient population (2.3±1.2) (Table 2). The authors conclude that treatment with MTX has changed over the last decade, and involves patients who have a shorter disease duration and less radiological damage. The doses are now higher and the clinical and radiological outcomes have improved. However, their analysis dealt with patients who continued to receive MTX over 24 months and excluded those who discontinued treatment before completing the 24 months because of secondary effects or lack of efficacy. Thus, as the authors mention, there could be a bias toward a better response to MTX (Table 3) (LE 4).
LeflunomideThe open-label RCT of Asaduzzaman et al.,17 carried out in 2014, compares the efficacy and safety of LEF and MTX in 32 PsA patients in Bangladesh (Table 1). Efficacy was primarily measured by the proportion of patients who met the response criteria at 6 months. To evaluate this, the authors utilized the ACR improvement criteria (ACR20, ACR50 and ACR70) and the PASI. All of the patients of the 2 groups achieved the primary objective but, for the secondary goal, there was a significant difference in ACR70 in favor of LEF (Table 2). It was concluded that LEF appears to be as effective and safe as MTX in PsA. However, in this open-label RCT, the sample size was small (Table 3) (LE 2b).
The prospective observational study of Asiri et al.,18 also performed in 2014, evaluated the efficacy and safety of LEF alone and in combination with MTX in 85 PsA patients (Table 1). Efficacy was defined by the continued administration of the drug, a reduction of ≥40% in TJC (TJC 40), a reduction of ≥40% in SJC (SJC 40) and PASI 50 and PASI 75 responses. The results between the 2 groups were similar (Table 2). It was concluded that LEF achieved an improvement in at least 50% of the patients at 1 year, and concomitant treatment with MTX was predictive of achieving PASI 50 at 12 months (odds ratio 6.19; 95% confidence interval, 0.20–31.97). In all, 30 patients (35%) interrupted LEF. The results were based on the patients who were taking the drug at any given moment of the study. However, if the response had been calculated on the basis of an intention-to-treat analysis, the proportion of responses could have been much lower (Table 3) (LE 4).
The objective of the multinational, prospective, observational study of Behrens et al.,19 published in 2013, which recruited 514 patients, was to determine the clinical efficacy and safety of LEF in adult patients with active PsA who were evaluated at 12 and 24 weeks. The primary outcome measure was the evaluation of the response according to the PsARC response. Other evaluations included global assessments, fatigue, pain, skin involvement, dactylitis and nail lesions (Table 1). There was significant improvement in counts like the TJC and SJC and in scores including physician and patient global assessments, as well as fatigue, pain, skin disease, dactylitis (from 46.7% at the baseline visit to 51.2% [n=467] in the last visit) and nail lesions (n=466 [32%]) (Table 2). It was concluded that LEF is an effective and well-tolerated option for the treatment of PsA in routine clinical practice, with beneficial effects on peripheral arthritis and on other symptoms mentioned above. In all, 12.3% of the patients (n=63) interrupted the treatment (98 adverse reactions in 62 patients [12.1%]; 3 serious [2 cases of increased liver enzymes and 1 hypertensive crisis]). Nevertheless, in comparison with a RCT, this study deals with routine clinical practice, in a diverse population of patients, with a wide variety of comorbidities and concomitant medication, which could be a potential confounding factor (Table 3) (LE 4).
The SR was not pursued beyond the qualitative synthesis and, as a result, it was not possible to perform a meta-analysis, as the number of studies retrieved was insufficient and due to the heterogeneity of the design of the articles included.
DiscussionThe results of the present SR, which is part of the Clinical Practice Guidelines for the Treatment of Axial Spondyloarthritis and Psoriatic Arthritis,12 are in accordance with the findings of the 2 previous SR included2,5; the first determined that there is little evidence of the efficacy of DMARDs in PsA and the second concluded that traditional DMARDs are utilized for the treatment of PsA, although the basis for the evidence of their efficacy is not well established.5 These results are also supported by the fact that similar to the findings in the 2009 Espoguia,21 it could be said that the present SR reveals diverse and asymmetrical evidence from the studies: specifically, with respect to the evaluated outcomes; we should mention that only 1 study took into account radiological damage20 and none of them commented on functional status or quality of life.
The studies involving LEF had similar results when compared. Thus, in the open-label RCT by Asaduzzaman et al.,17 LEF had some degree of efficacy and safety in PsA, as did MTX, since the primary objective was achieved in both groups; in the secondary outcome, there was a significant difference in ACR70 in favor of LEF. Likewise, in the observational study of Asiri et al.,18 LEF resulted in improvement in at least 50% of the patients, whereas concomitant treatment with MTX is shown to be a predictor of achieving the PASI 50 response. Likewise, the observational study published by Behrens et al.19 indicates that treatment with LEF is an effective alternative in the management of peripheral PsA, with a beneficial effect on pain, fatigue and dactylitis. On the other hand, although MTX is the DMARD most frequently utilized in PsA (39%),22 this could be because of the clinical benefit of MTX in cutaneous psoriasis.1 For example, in the study of Asiri et al.,18 a higher percentage of patients from the group taking a combination of MTX and LEF obtained a PASI 50 response, in comparison to the group receiving LEF as monotherapy.
However, there is a lack of evidence on treatment with MTX with respect to other DMARDs in the management of PsA, as occurred in previous reviews.23 The attempt has been made to explain this situation by different authors with a number of hypotheses. Thus, according to some authors, it could be due to the fact that the evidence of the treatment is supported, like that of the outcome measures,24 by its previous utilization in rheumatoid arthritis. Moreover, it can be considered that the number of well-designed clinical trials with relevant outcomes, as well as that of observational studies, is insufficient for the evaluation of the efficacy and safety of DMARDs in PsA.1,18,25,26
This same argument is stressed by Pincus et al.,27 as well as by other authors,28 the former group in a recent analysis on treatment with MTX, in which they evaluate the design of the study, among other causes, in the attempt to explain the influence of a number of factors on the result and on the quality of the evidence; they explore several hypotheses based on the results of the MIPA study, with different theories in terms of the high proportion of drop outs, a relatively low MTX dose and less severe inclusion criteria for PsA and fewer active joints.15,29
As an outcome of the proposed scenario, although studies may be included that result in the failure to achieve the best quality, as the absence of evidence does not imply evidence of absence.30 In agreement with a SR that evaluates the quality of 6 recent clinical guidelines on the treatment of PsA (with the exclusion of biological agents), the consequence would indicate that clinical practice guidelines have sufficient quality. The process of their development intrinsically involves objectivity and they are assessed in clinical practice, although it is considered that there could be some improvement in certain domains or items.26 One important limitation in this SR is the failure to perform a meta-analysis, as the number of studies retrieved was inadequate and their design was highly diverse, a drawback pointed out by Ceponis and Kavanaugh,1 among others. In accordance with the conclusions of the studies and SR evaluated,2,20 although, as suggested by Sevrain et al., it is unlikely that additional RCT be carried out because of the lack of interest in studying old drugs.26 We could conclude by proposing the need to conduct RCT or high-quality observational studies, with outcomes appropriate for the “core sets” proposed by OMERACT.31 They should adequately evaluate the efficacy of DMARDs according to the profile of clinical heterogeneity of PsA and support this indication, to improve the consistency of the level of evidence and, thus, reduce the possible uncertainty involving the therapeutic decision regarding early treatment.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of InterestThe authors declare they have no conflicts of interest.
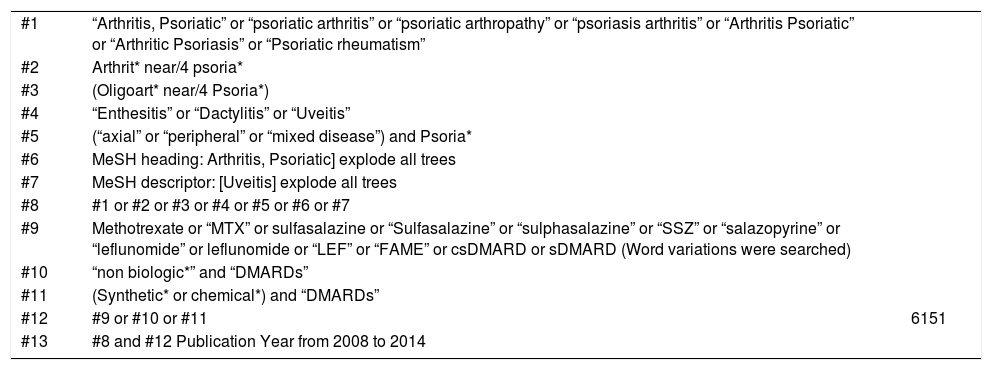
Cochrane Central: 97 results
| #1 | “Arthritis, Psoriatic” or “psoriatic arthritis” or “psoriatic arthropathy” or “psoriasis arthritis” or “Arthritis Psoriatic” or “Arthritic Psoriasis” or “Psoriatic rheumatism” | |
| #2 | Arthrit* near/4 psoria* | |
| #3 | (Oligoart* near/4 Psoria*) | |
| #4 | “Enthesitis” or “Dactylitis” or “Uveitis” | |
| #5 | (“axial” or “peripheral” or “mixed disease”) and Psoria* | |
| #6 | MeSH heading: Arthritis, Psoriatic] explode all trees | |
| #7 | MeSH descriptor: [Uveitis] explode all trees | |
| #8 | #1 or #2 or #3 or #4 or #5 or #6 or #7 | |
| #9 | Methotrexate or “MTX” or sulfasalazine or “Sulfasalazine” or “sulphasalazine” or “SSZ” or “salazopyrine” or “leflunomide” or leflunomide or “LEF” or “FAME” or csDMARD or sDMARD (Word variations were searched) | |
| #10 | “non biologic*” and “DMARDs” | |
| #11 | (Synthetic* or chemical*) and “DMARDs” | |
| #12 | #9 or #10 or #11 | 6151 |
| #13 | #8 and #12 Publication Year from 2008 to 2014 |
Medline (PubMed): 433 results
(“Arthritis, Psoriatic”[Mesh] OR “psoriatic arthritis”[Title/Abstract] OR “psoriasis arthritis”[Title/Abstract] OR “Arthritis Psoriatic”[Title/Abstract] OR “Arthritic Psoriasis”[Title/Abstract] OR (Arthritis[Title/Abstract] AND psoriatic[Title/Abstract]) OR (Oligoart* AND “Psoria*”) OR “Enthesitis”[Title/Abstract] OR “Dactylitis”[Title/Abstract] OR “Uveitis”[Mesh] OR ((“axial”[Title/Abstract] OR “peripheral”[Title/Abstract] OR “mixed disease”[Title/Abstract]) AND Psoria*)) AND (methotrexate[All Fields] OR “MTX”[Title/Abstract] OR sulfasalazine[All Fields] OR “Sulfasalazine”[Mesh] OR “sulphasalazine”[All Fields] OR “SSZ”[Title/Abstract] OR “salazopyrine”[All Fields] OR “leflunomide”[Substance Name] OR leflunomide[All Fields] OR “LEF”[Title/Abstract] OR “FAME”[Title/Abstract] OR (“non biologic*”[Title/Abstract] AND “DMARDs”[Title/Abstract]) OR ((Synthetic*[Title/Abstract] OR chemical*[Title/Abstract]) AND “DMARDs”[All Fields]))
NOT (“Animals”[Mesh] NOT (“Animals”[Mesh] AND “Humans”[Mesh]))
Filters: Publication date from 2008/01/01 to 2014/12/31; English; French; Spanish
EMBASE: 1132 results
‘arthritis, psoriatic’:ab,ti OR ‘psoriatic arthritis’:ab,ti OR ‘psoriatic arthropathy¿’:ab,ti OR ‘psoriasis arthritis’:ab,ti OR ‘arthritis psoriatic’:ab,ti OR ‘arthritic psoriasis’:ab,ti OR (arthr*:ab,ti AND psoria*:ab,ti) OR ‘psoriatic rheumatism’:ab,ti OR (oligoart*:ab,ti AND psoria*:ab,ti) OR ‘enthesitis’:ab,ti OR ‘dactylitis’:ab,ti OR ‘uveitis’:ab,ti OR (‘axial’:ab,ti OR ‘peripheral’:ab,ti OR ‘mixed disease’:ab,ti AND psoria*:ab,ti) AND (‘methotrexate’/exp OR methotrexate:ab,ti OR ‘mtx’/exp OR ‘sulfasalazine’/exp OR ‘sulfasalazine’:ab,ti OR ‘sulphasalazine’/exp OR ‘sulphasalazine’:ab,ti OR ‘ssz’:ab,ti OR ‘salazopyrine’/exp OR ‘salazopyrine’:ab,ti OR ‘leflunomide’:ab,ti OR ‘leflunomide’/exp OR leflunomide:ab,ti OR ‘fame’:ab,ti OR csdmard:ab,ti OR sdmard:ab,ti OR (‘non biologic’:ab,ti AND dmards:ab,ti) OR (synthetic*:ab,ti OR chemical*:ab,ti AND dmards:ab,ti)) NOT (‘animals’/exp NOT (‘animals’/exp AND ‘humans’/exp)) AND [embase]/lim NOT [medline]/lim AND ([english]/lim OR [french]/lim OR [spanish]/lim) AND [2008–2014]/py
Please cite this article as: Maese J, Díaz del Campo P, Seoane-Mato D, Guerra M, Cañete JD. Eficacia de los fármacos antirreumáticos modificadores de la enfermedad sintéticos en artritis psoriásica: una revisión sistemática. Reumatol Clin. 2018;14:81–89.