(1) To systematically and critically review the evidence on the characteristics, efficacy and safety of glucocorticoids (CS) in rheumatoid arthritis (RA); (2) to generate practical recommendations.
MethodsA systematic literature review was performed through a sensitive bibliographic search strategy in Medline, Embase and the Cochrane Library. We selected randomized clinical trials that analyzed the efficacy and/or safety of CS in patients with RA. Two reviewers performed the first selection by title and abstract. Then 10 reviewers selected the studies after a detailed review of the articles and data collection. The quality of the studies was evaluated with the Jadad scale. In a nominal group meeting, based on the results of the systematic literature review, related recommendations were reached by consensus.
ResultsA total of 47 articles were finally included. CS in combination with disease-modifying antirheumatic drugs help control disease activity and inhibit radiographic progression, especially in the short-to-medium term and in early RA. CS can also improve function and relieve pain. Different types and routes of administration are effective, but there is no standardized scheme (initial dose, tapering and duration of treatment) that is superior to others. Adverse events when using CS are very frequent and are dose-dependent and variable severity, although most are mild. Seven recommendations were generated on the use and risk management of CS.
ConclusionsThese recommendations aim to resolve some common clinical questions and aid in decision-making for CS use in RA.
1) Revisar sistemática y críticamente la evidencia sobre las características de uso, eficacia y seguridad de los glucocorticoides (GC) en la artritis reumatoide (AR); 2) emitir recomendaciones prácticas sobre su utilización.
MétodosSe realizó una revisión sistemática de la literatura con una estrategia de búsqueda bibliográfica sensible en Medline, Embase y Cochrane Library. Se seleccionaron ensayos clínicos aleatorizados que analizasen la eficacia y/o la seguridad de los GC en pacientes con AR. Dos revisores realizaron la primera selección por título y abstract y 10, la selección tras lectura en detalle y la recogida de datos. La calidad se evaluó con la escala de Jadad. En una reunión de grupo nominal con base en sus resultados se consensuaron una serie de recomendaciones.
ResultadosSe incluyeron 47 artículos. Los GC, en combinación con los fármacos antirreumáticos modificadores de la enfermedad, ayudan a controlar la actividad de la enfermedad y a inhibir la progresión radiográfica, especialmente en el corto-medio plazo y en las AR de inicio. Los GC pueden mejorar la función y el dolor. Distintos tipos y vías de administración son eficaces, sin que exista un esquema de tratamiento estandarizado (dosis de inicio, desescalada y duración del tratamiento con los GC) superior a otro. Los acontecimientos adversos de los GC son muy frecuentes, dependientes de la dosis, de gravedad variable, muchos de ellos leves. Se generaron 7 recomendaciones sobre el uso y la gestión del riesgo de los GC.
ConclusionesEstas recomendaciones pretenden resolver algunos interrogantes clínicos habituales y facilitar la toma de decisiones con respecto al uso de GC en la AR.
Glucocorticosteroids (GCC) are one of the most broadly used therapies in the field of rheumatology,1–4 and their efficacy and safety profiles have been extensively described.5–10 It has been demonstrated that they are able to help control the inflammation and symptoms of these diseases. However, their mechanism of action, effectivity and role within the therapeutic strategy of the majority of immune mediated diseases is very different compared, for example, with disease modifying anti-rheumatic drugs (DMARDS). Equally, their safety profile and particularly in the medium and long term or with high dose usages has led to major doubts because they are is associated with relevant adverse events (AE) such as osteoporosis or cardiovascular risk.
However, in the specific case of rheumatoid arthritis (RA), one of the first events taking place in 1949 was when Philip Hench reported a spectacular clinical effect after administrating GCC in patients with RA.11 Although since then the therapeutic arsenal has increased enormously with the use of synthetic DMARDS and the subsequent arrival of biologic therapies, GCC are still a highly used therapy in RA.12,13
Curiously, over 60 years later, doubts remain regarding the precise role currently played by GCC in RA management. There is some variability in the recommendations given by scientific societies, both national and international. In all of them their use is recommended in early RA as a coadjuvant treatment with synthetic DMARDS.14–19 However, not all of them refer to use in established RA. Some recommend use in this type of patient as symptomatic treatment,15 or when a DMARD or biologic agent is unsuccessful,14 or when there are reactivations.17 From that stage onwards indications are often vague, particularly with regard to dose, time periods and suspension, although in general the use of GCC at the lowest possibly dose is recommended, aimed at gradual dose tapering until suspension, in the shortest possible time.14,16,18,19 Regarding the definition of low dose, on occasions the simple indication of “low dose”16 is present; in others it is defined as ≤10mg/day, as in the ACR document,14 or 7.5mg/day in that of EULAR.19 Regarding length of time, we may find, for example, recommendations for temporal use (<6 months),18 or short cycles on initiation or changing DMARD.19 The APLAR guide includes higher doses for extra-articular manifestations,17 and the EULAR guide includes local infiltrations with GCC for local inflammation symptoms,18 and also the only intramuscular dose (120mg of methylprednisolone) and the only intravenous dose (250mg of methylprednisolone).19 The PANLAR document of consensus on RA management, however, does not mention GCC usage.20
Based on the above, and in the context of the NEXUS Project, the aim of this systematic review was basically to analyse the efficacy and safety of GCC usage in RA so as to issue a series of practical recommendations that would serve as a guide for clinicians in their daily practice.
MethodsNEXUS projectThis publication forms part of the NEXUS Project, comprising 2 national coordinators, 8 work groups with a regional coordinator for each and 2 or 3 reviews (depending on the group), for a total of 22 reviewers. Each year different subjects of interest in the RA area are analysed. Those of this issue were the use of GCC and their combined therapy with synthetic DMARDS in RA. The Spanish Society of Rheumatology guarantees that the methodology used is appropriate, but does not endorse the conclusions, since there are official bodies that do this.
Review protocolInitially, the coordinator put forward the following questions which were responded to through an SLR: What is the effective GCC dose? (dose at which the RA activity falls and may even be totally controlled, or other efficacy parameters); At what dose (and/or cumulative dose) of GCC do AE appear?; what type of AE?; What are low doses of GCC? (the definitions offered by the articles, particularly if they defined them); what is the pattern, outline, protocol of GCC usage in RA? (early RA, established RA or outbreaks, what dose should be started with, maintained, how should the dose be reduced, in what time periods, etc.). An SLR protocol was defined with these questions.
PICO and study selection criteriaThe previously mentioned questions were transformed into the PICO with which the inclusion and exclusion criteria were defined. We selected studies which included patients with RA (international criteria or clinical judgement), adults (≥18 years), regardless of the disease duration or previous treatments –P–; in treatment with GCC –I– with or without DMARDS (synthetic or biologic), regardless of the type, dose, treatment regime, administration route, etc. As a comparator –C–, the studies could use placebo or an active comparator (NSAID, DMARD, etc.). Also, articles were sought in which their outcomes –O analysed the features of the GCC used, the dose and/or time of use of GCC where efficacy occurred (any parameter used regularly to measure clinical efficacy in RA), dose and/or time of use of GCC with AE and types of AE. Studies were included which defined the following: low dose of GCC, cumulative dose and effects on the patient, etc. Finally, only those studies with the following designs were included: metaanalysis, systematic reviews and randomised clinical trials (RCT). Studies on animals and basic science were excluded.
Search strategyAided by an expert documentalist search strategies were created for the different databases. For this they used the MeSH terms and terms in free text. Only articles on humans, in English or Spanish were included in the search.
For this review the following bibliographic data bases were sifted: Medline, Embase and Cochrane Library (all from their initiation up until July 2017). Due to the volume of bibliographic references recovered, we decided not to review the grey literature of the main national and international rheumatology conferences. A manual search was subsequently performed secondary to the bibliography of the articles finally included. The supplementary material shows the search strategy used in Medline, Embase and Cochrane, together with the number of references collected.
All the references resulting from the searches were inserted into the EndNote programme for easier management.
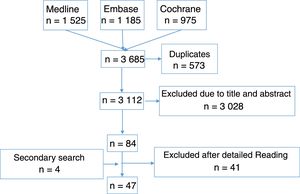
Article selectionFollowing this, 2 reviewers created the first selection of articles resulting from the search strategy by reading the title and abstract, complying with the inclusion and exclusion criteria, each independently. Whenever a discrepancy arose, a third reviewer was taken on board to make a decision. After this, 10 reviewers made a second article selection through independent detailed reading and applying the same inclusion and exclusion criteria. To do this, the number of references collected was equally distributed among the 10 reviewers. Furthermore, one of the reviewers from the previous stage also reviewed all the articles of this stage acting as the comparator of the 10 reviewers. Whenever a discrepancy arose, the other reviewer of the previous phase resolved the problem. In Fig. 1 we show the flow diagram of the selection process of the articles, and in the supplementary material, the characteristics of the studies included and excluded.
Data collection and assessment of the study qualityThe 10 reviewers and one of the reviewers from the first selection stage, collected the study data included using specifically pre-designed templates for this review (CRD). The Jadad21 scale was used to assess the methodological quality of the studies included. Again, where discrepancies arose the other reviewer from the previous stage resolved the problem.
Data analysis and presentationTables of evidence and outcomes were created, where the main characteristics and outcomes of the included studies were described. Some of these were expressed as numbers and percentages, mean and standard deviation, median and interquartile range (p25–p75); others as odds ratios, relative risk or hazard ratios and their 95% confidence intervals. The possibility of performing a metaanalysis was only assessed where there was homogeneity.
Nominal group meeting and drawing up of recommendationsDuring a 2-day nominal group meeting which all NEXUS project members attended, the outcomes of the SLR were presented and discussed. A series of recommendations were agreed to. Each of the recommendations, with guidance from methodology, was assigned a level of evidence and a level of recommendation, in keeping with the recommendations for evidence-based medicine from the Centre for Evidence-Based Medicine in Oxford.22
ResultsThe search strategies collected 3112 references (Fig. 1), of which finally 47 RCT23–69 were included, of variable quality (Jadad 1–5), most of them moderate. Duration was also variable, from 12 weeks27 to 4 years.39 The oldest article was from the year 1954 and the most recent was that published in 2016. Over 5000 patients with RA were analysed, both from disease onset23,25,55,58 and when the disease was established.26,27,29,40,64 Regarding the outcome variables analysed and their tools, RA activity was included in the studies (number of joints, composite benchmarks, morning stiffness), function (Health Assessment Questionnaire), radiographic damage (Sharp van der Heijde, Sharp, Larsen, Ratingen), quality of life (SF-36, EuroQol), pain (visual analogue scale), sleep quality (e visual analogue scale), overall evaluation of the patient and overall evaluation of the doctor with the visual analogue scale, or variables relating to their employment.
Table 1 summarises the conclusions and recommendations of the SLR. Supplementary material may also be consulted.
Main conclusions and recommendations from the review.
| Conclusions | |
|---|---|
| 1 | Low doses of corticosteroids are considered to be those equal to or below 7.5mg/day of Prednisone or its equivalent (NE 5; RL D) |
| 2 | Corticosteroids combined with DMARDS help to control the disease activity and inhibit radiographic progression, particularly in the short to medium term and at the start of RA (EL 1b; EL A) |
| 3 | Corticosteroids may improve other variables such as function and pain (EL 1b; RL B) |
| 4 | Different types of corticosteroids and administration routes are effective (EL 1a; RL A) |
| 5 | With the available evidence it is not possible to define a standardised guideline which is clearly superior in efficacy/safety ratio for the initial dose, tapering and treatment duration of the corticosteroids (EL 5; RL D) |
| 6 | AE are very common with corticosteroid usage, depending on dose and time of administration and their severity is highly variable (EL 1a; RL A) |
| Recommendations | |
| 1 | Patients with early RA are recommended to be treated with corticosteroids combined with synthetic DMARDS EL 1b; RL A) |
| 2 | In established RA the individualised use of corticosteroids is recommended as symptomatic treatment (EL 5; RL D) |
| 3 | The use of corticosteroids at low doses is recommended as a bridge therapy in early RA (≤7.5mg/day of Prednisone or equivalent) and at intermediate doses (30mg/day) in rapidly descending regime (EL 1b; RL A) |
| 4 | In patients with RA the use of corticosteroids at the lowest effective dose and for the shortest possible time is recommended (EL 1b; RL B) |
| 5 | If corticosteroids are to be used in patients with RA it is recommended that the patients be assessed prior to initiation of treatment to rule out comorbidities and the risk of infection (EL 5; RL D) |
| 6 | If corticosteroids are prescribed in the medium to long term in patients with RA it is recommended that strict monitoring of cardiovascular risk factors and bone mineral density factors be undertaken (EL 5; RL D) |
| 7 | If corticosteroids are prescribed in the medium to long term in patients with RA prophylaxis for osteoporosis induced by corticosteroids is recommended (in compliance with international guidelines) (EL 5; RL D) |
RA: rheumatoid arthritis; DMARDS: disease-modifying anti-rheumatic drugs; RL: recommendation level; EL: evidence level.
There is no universally accepted definition of low GCC dose. Depending on the study, doses of up to15mg/day5,6: are generally considered: 5mg/day,68,70 6.25mg/day,51,71 7mg/day,28 7.5mg/day,23,24,33,47,54,72,73 10mg/day,48,57,74,75 15mg/day.76,77 In one study doses of mg/day or lower were considered very low doses.61
GCC usage characteristicsThose most commonly prescribed were prednisone and prednisolone, although others mentioned were methylprednisolone, dexamethasone, cortisone acetate or deflazacort. They were mainly administered orally but also intramuscularly29 and even in intravenous boluses23 or as an intraarticular infiltration.40,49
They were used in monotherapy or combined with other DMARDS (in turn as monotherapy23–25,41,51,58,60 or combined23,25,32,49,62), at highly varied doses, both as early and as maintenance therapy. The initial dose varied from <15mg/day6 to a minimum of 1–4mg/day52; some studies began with higher doses of 60mg/day and gradually lowered the dose to 7.5mg/day.25,36 Maintenance doses were also highly variable, but were generally <7.5mg/day.
Regarding treatment regimes, different types have been published. In general, using higher doses at the beginning and then reducing them was also carried out with higher doses, with reductions described of up to 10mg/week. The lowest descending dose found was 2.5 2,5mg/week. In many studies the dose is progressively reduced until it reaches what is considered to be the minimum effective dose (generally undefined criterion).34,35,78Table 2 describes the principal regimes found in the SLR.23,25,30,31,36,38,48,51,53,55,58–60,64,79
Treatment regimes with corticosteroids.
| 1 | Prednisolone, 1st week 60mg/day, 2nd week 40mg/day, 3rd week 25mg/day, 4th week 20mg/day, 5th week 15mg/day, 6th week 10mg/day, 7th week 7.5mg/day. Some authors consider this guideline to be the classical COBRA strategy |
| 2 | This is, according to the authors, a light COBRA strategy. Prednisolone week 30mg/day, 1st week 27.5mg/day, 2nd week 25mg/day, 3rd week 22.5mg/day, 4th week 20mg/day, 5th week 17.5mg/day, 6th week 15mg/day, 7th week 12.5mg/day, 8th week 10mg/day, 9th week 7.5mg/day |
| 3 | This is included in the COBRA Slim and COBRA Avant-Garde strategies. Prednisolone week 30mg/day 1st week, 20mg/day 2nd week, 12.5mg/day 3rd week, 10mg/day 4th week, 7.5mg/day 5th week, 5mg/day 6th week |
| 4 | Prednisone 60mg/day 1st week→40mg/day 2nd week→25mg/day 3rd week→20mg/day 4th week→15mg/day 5th week→10mg/day 6th week→7.5mg/day until week 28, with subsequent removal from one day per week of Prednisone, 2 days per week, 3 days per week, 4 days per week, 5 days per week, 6 days per week and 7 days per week, until total suspension on week 35 |
| 5 | Prednisolone 60mg/day initially, reduced to 7.5mg/day at 6 weeks, 7.5mg/day from week 6 to 28, and suspended at week 34 |
| 6 | Prednisone 60mg/day for one week, descending to 7.5mg/day in week 7–28 and finally reduced until suspension at week 36 |
| 7 | Prednisone 20mg/day (days 1–5), 10mg/day (days 6–10), 5mg/day (days 11–14), and then adjusted according to disease activity |
| 8 | Prednisone 30mg/day for 15 days→20mg/day for 15 days→up to 2.5–15mg/day to control RA activity |
| 9 | Prednisone 20mg/day (days 1–5), 10mg (days 6–10), 5mg/day and then adjusted depending on medical symptoms |
| 10 | Prednisone 15mg/day one month, if clinical response (according to patient criterion) reduced 2.5mg/day at intervals of 4 weeks until minimum effective dose |
| 11 | Prednisone 20mg/day 15 days→10mg/day 90 days |
| 12 | Deflazacort 24mg/day 15 days→13mg/day 90 days |
| 13 | Prednisone 10mg/day for 12 weeks and reduction to 7.5mg/day on weeks 13 and 14, then 5mg/day weeks 15 and 16, to 2.5mg/day weeks 17 and 18 to suspend on 19 and 20 |
| 14 | Prednisone 12.5mg/day for 2 weeks, with progressive reduction (non specified guideline) to 6.25mg/day |
RA: Rheumatoid Arthritis.
With regard to RA activity, GCC offer greater control compared with placebo or NSAIDS (such as ibuprofen or ASA) at least in the short to medium term.6,27,42,43,65,66 Also, their combination with synthetic DMARDS (monotherapy or combined therapy), in early RA (≤2 years), achieves greater and faster improvement,23,25,51 although in established RA they also help in to control the disease activity.26,27,29,40,64 This control is achieved even with low doses (≤7.5mg/day of prednisone or equivalent)27 and from the first month (rapid effect),29 although the intermediate GCC doses (20–30mg/day prednisone or equivalent)60 or high dose (60mg/day) with fast dose tapering30,36 have also proven to be effective as a bridge therapy. Long term data also prove clinical efficacy.30,39,54 One alternative to oral GCC with rapid dose reduction may be an intramuscular injection of 120mg of methylprednisolone or an intravenous bolus of 250mg.23,29 Intraarticular infiltrations may also help to control disease activity in patients taking DMARDS.38,40,49 Finally, in terms of disease activity control, it has not yet been proven that any one initial dose, tapering or maintenance dose is better than any other,31 although it has been seen that in RA controls, a maintenance dose of 5mg/day produces a positive effect.52
Analysis of radiographic damage progression shows that the GCC help the DMARDS in inhibiting this for at least the first 2–3 years of treatment7,23,24,29,30,36,37,53,54,57,61; some studies show that this protective effect continues for up to 4–5 years,39 both with medium-high doses at onset25,30,53 and with low ones (<10mg/day).24,39,54,57,61 However, it should be noted that not all RCT confirm their ability to prevent radiographic damage.58,59
Regarding function, the use of GCC at the onset of RA together with DMARDS helped to improve it especially in the short term,26,29,30,36,37,54 where the improvement is faster (better than if they are not combined), both with a medium-high initial dose (15–60mg/day)30,36,37 and low one (≤10mg/day).26,39,54 In the long term it may also positively contribute for up to 4 years.23,24,39
It was also noted that in patients with controlled RA, low maintenance doses (<5mg/day) could contribute to pain control.5 Quality of life may improve with the use of GCC,26,30,53 but their efficacy in relation to other variables such as the overall physician assessment, overall patient assessment or factors related to employment have not currently been analysed to any great extent.29,52 Similarly to RA activity control, no initiial dose, tapering regime or maintenance dose exists that is any better than any other.
SafetyThe presentation of AE with GCC use (of any type and administration route) in patients with AR is very common. The rate of any AE varies from under 50%27 to almost 100% depending on the article.31 The majority are dose-dependent and (many) may appear with very short GCC cycles.27,32,37,38,44,49 However, the rate of severe AE is low, generally lower than 5%, deaths are exceptional and the rate of discontinuation is also low, at least in the short term.3,24,26,27,29,30,43,44,53
The most common AE particularly in the short term are digestive (nausea, vomiting, dyspepsia, gastritis, etc.), headache, anxiety, high blood pressure, hyperglycaemia and skin disorders27,31,32,36–38,43,44,49; in the medium-long term, osteoporosis, infections, cardiovascular events and other cardiovascular risk factors.28–30,36,43,44,50,51,53,54,57,60,61
However, with regard to bone metabolism and osteoporosis, there is a clear association with a reduction in bone mineral density at lumbar and hip level (less clear, it depends on the study),33,80 dose-dependent, the process of which begins from the start of treatment.81 Long-term use is associated with vertebral fractures even in low doses (<10–15mg/day).6
With regard to cardiovascular risk, GCC increase the risk of any cardiovascular event (relative risk of 1.47; 95% confidence risk 1.34–1.60; P<.001), and also of AMI, stroke, heart failure and greater cardiovascular AE.82,83
Regarding infections, based on the RCT data, no clear association with them was observed, possibly due to their design, the type of patients included and the low number of AE of this type.84 However, in observational studs, this was clearly regarded as a risk factor for ingections,85,86 and especially in elderly patients.
Finally, it is impossible to determine whether doses of specific administration regimes imply an obvious improvement in terms of safety, except when the lowest possible dose is used and preventative treatment for osteoporosis.41
DiscussionAt present, GCC play a major role in RA patient management, endorsed by evidence and many years of medical experience.5–10 However, it is particularly their safety profile from which usage limitations arise.5–10 For this reason, the recommendations issued by national and international scientific societies are genially aimed at the use of GCC in RA at the lowest doses and for the shortest possible time.14,16,18,19
The conclusions expressed in this document aim to assist the clinician in the use of GCC, either strengthening (given their significance) the messages which have already been communicated, or specifying other aspects where further doubts could exist. To sum up, based on their proven efficacy, but also on their AE, we have recommended their use particularly for early RA, always at the lowest dose and for the shortest possible time. We would also like to point out that we have found no one initial dose, treatment regime or administration route to be better than any other.
This SLR has certain limitations. Despite the large number of RCT analysed, there was a huge variability between them partially relating to the quality of the studies and their contexts. Studies with half a century difference from one another were analysed, with huge inequalities regarding medical practice. Also the fact that GCC are used at different doses, administration routes, tapering regimes and for different time periods limits or complicates the standardisation of outcomes. Similarly, the concomitant use of other drugs in many studies may impact overall outcomes, making it difficult at times to estimate the true magnitude of the effects of the GCC. Moreover, RCT design does not always lead to precise assessment of the safety profile of the drugs, in this case the GCC.
However, despite these limitations and assisted by many years of experience in the use of GCC in RA we hope that the outcomes, conclusions and recommendations expressed in this article may be of positive guidance for the rational use of these drugs in RA.
FinancingThe NEXUS Project was financed by Roche, which neither participate in the selection of subjects nor in the development of this article, the conclusions or the recommendations.
Conflict of interestsThe authors have no conflict of interests to declare.
Our thanks to Roche, for their involvement in the NEXUS project. Also to the members of the NEXUS group for their participation in the review: Rosa Gonzalez Crespo and Alejandro Escudero. And to Doctors Liliana Ercole and Estíbaliz Loza, for their methodological and logistic coordination.
Please cite this article as: Sanmartí R, Tornero J, Narváez J, Muñoz A, Garmendia E, Ortiz AM, et al. Eficacia y seguridad de los glucocorticoides en la artritis reumatoide: revisión sistemática de la literatura. Reumatol Clin. 2020;16:222–228.