To review the efficacy and safety of rituximab in vasculitic neuropathy (VN).
MethodsA literature search was performed on Medline and Embase up until 2017. It included terms related to “vasculitis”, “vasculitic neuropathy” and “Rituximab”. Research was carried out by two reviewers. The main outcome was rituximab efficacy.
ResultsOf an initial selection of 702 articles, 5 remained with a level of evidence between 1+ and 3 and variable recommendation degree. In the only clinical trial included, rituximab was superior to conventional therapy for cryoglobulinemic vasculitis with VN showing an increase in drug retention rate (64.3% vs 3.5%; P<.001) and with a lower rate of serious adverse effects (.12 vs .48). Cohort studies of patients with cryoglobulinemic vasculitis showed improvement and complete/partial remission of VN. In a series of 5 cases of refractory EGPA suffering from VN, 60% and 20% of patients achieved complete and partial remission respectively.
ConclusionsRituximab seems an effective and safe treatment for VN in the context of cryoglobulinemic vasculitis. Evidence for specific efficacy in VN in the context of other types of vasculitis is lacking.
Revisar la eficacia y seguridad del rituximab en neuropatía vasculítica (NV).
MétodosSe realizó una búsqueda en la literatura de Medline y Embase hasta 2017. Los términos incluidos guardaron relación con «vasculitis», «neuropatía vasculítica» y «rituximab». Dicha búsqueda fue realizada por 2 revisores. El resultado principal fue la eficacia del rituximab.
ResultadosTras seleccionar inicialmente 702 artículos, 5 de ellos permanecieron con un nivel de evidencia de entre 1+ y 3, y un grado de recomendación variable. En el único ensayo clínico incluido, el rituximab fue superior a la terapia convencional para vasculitis crioglobulinémica, mostrando NV un incremento en la tasa de retención farmacológica (64,3 vs 3,5%; p<0,001) y una menor tasa de efectos adversos graves (0,12 vs 0,48). Los estudios de cohortes de pacientes con vasculitis crioglobulinémica incluyeron una mejora probada y una remisión completa/parcial de NV. En una serie de 5 casos de EGPA refractaria con NV, el 60% y el 20% de los pacientes lograron una remisión completa y parcial, respectivamente.
ConclusionesRituximab parece ser un tratamiento eficaz y seguro para NV, en el contexto de vasculitis crioglobulinémica. Se carece de evidencia sobre la eficacia específica de NV en el contexto de otras vasculitis.
The term vasculitis comprises a heterogenous group of diseases characterised by the inflammation and destruction of the blood vessel wall, which causes damage to different organs and tissues.1,2
The involvement of the peripheral nervous system caused by vasculitis, i.e., vasculitic neuropathy is the result of ischaemic damage by occlusion of the blood vessel. The thick sensitive and motor nerve fibres are usually compromised, as they are more susceptible to ischaemic damage and there is predominantly axonal compromise.3
The standard presentation of peripheral nervous system involvement by vasculitis is a painful multiple mononeuropathy (“multiplex mononeuritis”) although it may also be presented as rapidly progressive polyneuropathy. Its presentation as polyneuropathy dependent on chronic length4 is less common.
Vasculitic neuropathy is generally another manifestation of a systemic condition which may affect skin, lungs, kidneys and other organs. In a review of 106 patients with vasculitic neuropathy followed up for a period of 28 years,5 the main diseases which evolved with the vasculitic neuropathy were systemic vasculitis (eosinophilic granulomatosis with polyangitis, polyarteritis nudosa, granulomatosis with polyangitis, microscopic polyangitis, cryoglobulinaemia) and rheumatic diseases like rheumatoid arthritis or systemic lupus erythematosus. The frequency of vasculitic neuropathy for some of these diseases according to some studies6–9 would be 80% in granulomatosis with polyangitis and eosinophilic granulomatosis with polyangitis, 70% in microscopic polyangitis, 50–70% in polyarteritis nudosa and 70% in mixed cryoglobulinaemia. In a minority of patients with vasculitic neuropathy however, the vasculitis of the peripheral nervous system is produced in isolation as a non systemic vasculitic neuropathy.5,10–12
Despite the fact that vasculitic neuropathies are relatively uncommon, their identification and treatment is important because of the morbidity and incapacity they cause in patients.13 Even in the absence of systemic involvement, vasculitic neuropathy may lead to serious functional disabilities from which some patients never fully recover.
There is no specific treatment for vasculitic neuropathy, which depends on the context in which it occurs. E.g., immunosuppressive agents are used and glucocorticoids in primary vasculitis or vasculitis associated with systemic rheumatic diseases and antivirals in the cases of cryoglobulinaemia associated with hepatitis C.14,15
Rituximab (RTX) is a chimeric anti-CD20 monoclonal antibody with B cell depletion effects, which has been approved by the European Medicines Agency (EMA) for the treatment of rheumatoid arthritis, granulomatosis with polyangitis and microscopic polyangitis, in addition to the non-Hodgkin group of lymphomas and chronic lymphoid leukaemia.16 It has also been used outside of its technical specifications for systemic erythematosus, systemic sclerosis and other types of vasculitis such as mixed cryoglobulinaemia. Our objective was therefore to review the evidence on RTX efficacy in vasculitic neuropathy specifically and to study its possible adverse effects on these patients.
MethodsA systematic review of those studies which were able to respond to the research question was made.
Study Identification and SelectionAn exhaustive bibliographical search was undertaken in Medline and Embase from 1961 until October 2017, restricting the search of articles to English, French and Spanish relating to humans. The search strategy included the terms MeSH and the free text and terms relating to “vasculitis”, “vasculitic neuropathy” and “Rituximab”. Search strategies were made by 2 authors who separately reviewed the titles, abstracts and selection criteria.
The following were included depending on the study type: meta-analysis, systematic reviews, clinical trials and observational studies (cross-sectional, cohort and case-control study). Depending on the type of participant, studies with adult patients who had been diagnosed with vasculitic neuropathy were included. The use of any method validated for the diagnosis of vasculitis and the neuropathy may have been clinically diagnosed, through complementary examinations or through both. Patients with neuropathy of origin other than vasculitic were excluded. Depending on the type of intervention, selection was of studies which assessed the efficacy of treatment with RTX and which considered the use of RTX alone or in combination therapy, after a minimum follow-up interval of at least 6 months.
The main outcome measurements studied were efficacy with RTX, i.e., sensorial impairment at the end of the follow-up period (at least 6 months after treatment initiation), using any validated neuropathy scale or quantitative sensorial tests. Secondary outcomes included were: RTX treatment retention time; number of participants with improvement or resolution of neuropathy symptoms determined by the overall impression of the researcher or the patient evaluation scale; number of mild and serious adverse events during the follow-up period.
Data Extraction and Evaluation of BiasesThe articles whose titles or abstracts coincided with the inclusion criteria were read in their complete format. The lack of any one eligibility criteria was sufficient to exclude a study. Any disagreement regarding the inclusion of a study was resolved by consensus between the 2 reviewers with the help of a third reviewer. Two reviewers extracted the data from the documents separately as a report.
The quality of the evidence was analysed through a reading of the complete text following the guidelines of the Scottish Intercollegiate Guidelines Network (SIGN).
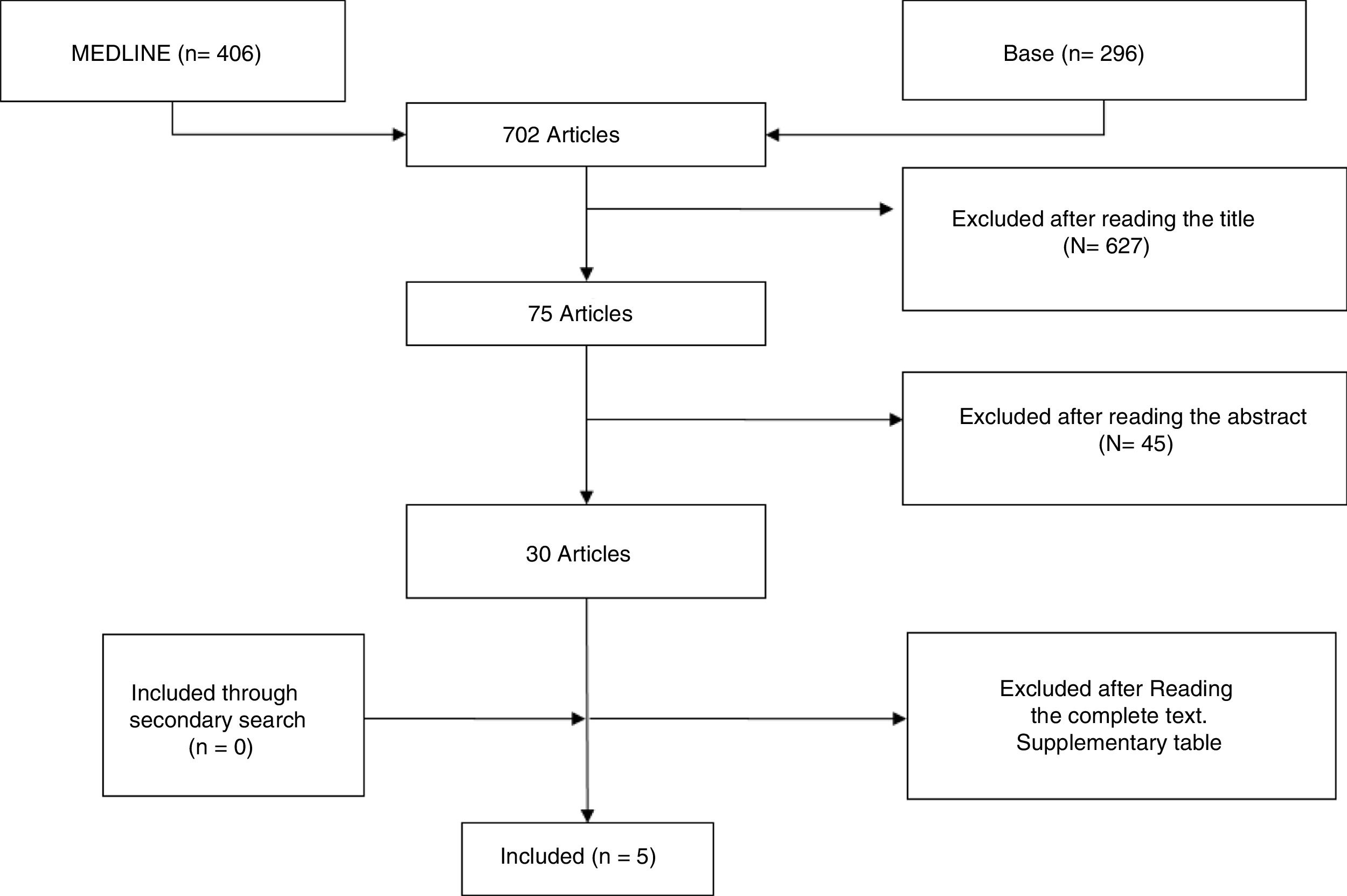
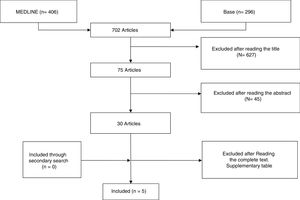
ResultsStudy DescriptionAfter selecting the 296 articles published between 1999 and 2017, 42 duplicates were eliminated, 224 were eliminated after reading the title and abstract and 25 after reading the complete article. Only 5 articles finally met with the established selection criteria and comprised the focus of this systematic review (Fig. 1). The studies included were: one clinical trial, 3 cohort studies and a case series (Table 1). The majority of studies were cohorts with a level of evidence between 1+ and 3 and a SIGN B recommendation level.
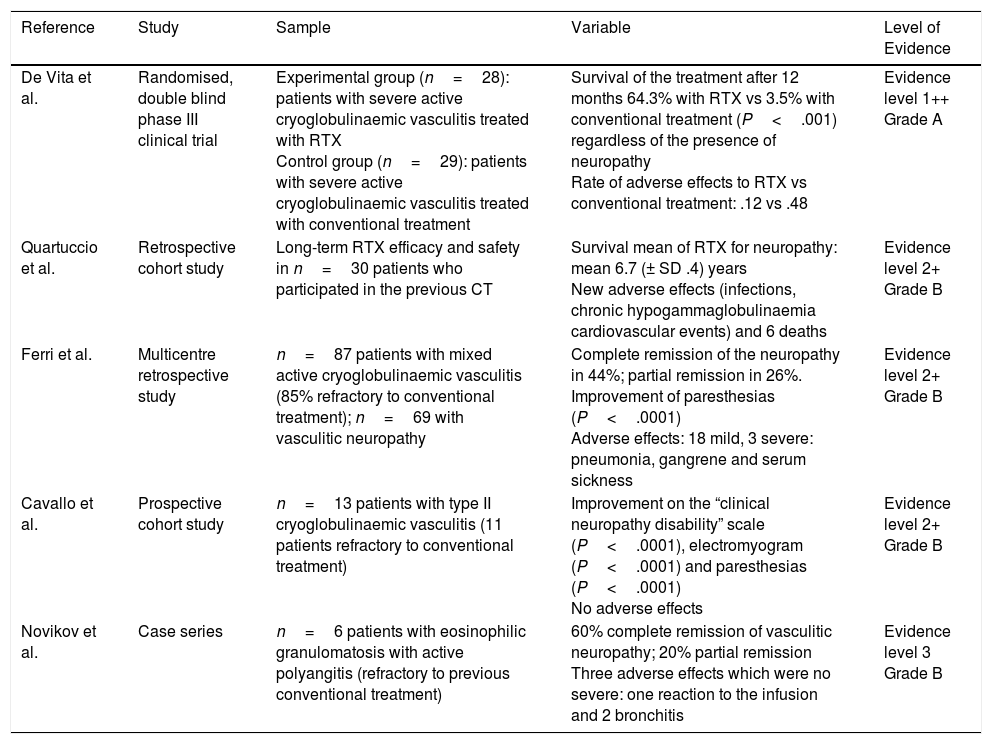
Studies Included.
| Reference | Study | Sample | Variable | Level of Evidence |
|---|---|---|---|---|
| De Vita et al. | Randomised, double blind phase III clinical trial | Experimental group (n=28): patients with severe active cryoglobulinaemic vasculitis treated with RTX Control group (n=29): patients with severe active cryoglobulinaemic vasculitis treated with conventional treatment | Survival of the treatment after 12 months 64.3% with RTX vs 3.5% with conventional treatment (P<.001) regardless of the presence of neuropathy Rate of adverse effects to RTX vs conventional treatment: .12 vs .48 | Evidence level 1++ Grade A |
| Quartuccio et al. | Retrospective cohort study | Long-term RTX efficacy and safety in n=30 patients who participated in the previous CT | Survival mean of RTX for neuropathy: mean 6.7 (± SD .4) years New adverse effects (infections, chronic hypogammaglobulinaemia cardiovascular events) and 6 deaths | Evidence level 2+ Grade B |
| Ferri et al. | Multicentre retrospective study | n=87 patients with mixed active cryoglobulinaemic vasculitis (85% refractory to conventional treatment); n=69 with vasculitic neuropathy | Complete remission of the neuropathy in 44%; partial remission in 26%. Improvement of paresthesias (P<.0001) Adverse effects: 18 mild, 3 severe: pneumonia, gangrene and serum sickness | Evidence level 2+ Grade B |
| Cavallo et al. | Prospective cohort study | n=13 patients with type II cryoglobulinaemic vasculitis (11 patients refractory to conventional treatment) | Improvement on the “clinical neuropathy disability” scale (P<.0001), electromyogram (P<.0001) and paresthesias (P<.0001) No adverse effects | Evidence level 2+ Grade B |
| Novikov et al. | Case series | n=6 patients with eosinophilic granulomatosis with active polyangitis (refractory to previous conventional treatment) | 60% complete remission of vasculitic neuropathy; 20% partial remission Three adverse effects which were no severe: one reaction to the infusion and 2 bronchitis | Evidence level 3 Grade B |
SD: standard deviation; CT: clinical trial; RTX: rituximab.
The excluded studies and reasons for exclusion are mentioned in additional table. The total number of patients with vasculitic neuropathy treated with RTX and included in this review were 123, ranging between 5 and 69 patients from one article to another. Regarding the duration of follow-up observed in the studies, this was variable, between 6 months and 6 years.
Previous TreatmentIn the majority of studies analysed, initiation of treatment with RTX was described after the failure with conventional treatment. In the study by Cavallo et al.17 11 out of 13 patients were refractory to prior treatment (all to corticoids and cyclophosphamide, micophenolate, plasmapheresis and interferon). In the Novikov et al.18 study in 6 patients with granulomatosis with eosinphilic polyangitis treated with RTX all of them were refractory to standard treatment (all to corticoids and cyclophosphamide and others also to azatioprine, methotrexate and micophenolate in combination therapy). In the Ferri et al.19 study only 15% of patients received RTX as first line treatment with the others being refractory to standard treatment.
In the clinical trial by De Vita et al.20 and the posterior long-term retrospective study on the same patients by Quartuccio et al.21 there was no reflection as to whether there had been treatment prior to the randomisation for cryoglobulinaemic vasculitis. They only reflected that the patients with positive serology of the hepatitis C virus were not treated with antiviral agents or if they had been, the agents were ineffective.
Treatment Regimen With RituximabIn all the studies RTX was used in monotherapy (not counting low dose corticoids), except in the study by Novikov et al.18 where treatment with RTX was combined with baseline treatment in one patient (azatioprine).
In the majority of studies the total dose of RTX (2g) was administered in weekly or two-weekly intravenous infusions for one month and in all of the studies the doses from 6 months onwards were on demand, depending on the clinical situation. Ten of the 87 patients from the Ferri et al.19 study and all the patients from the Cavallo et al.17 study received a cycle of 6 infusions at 375mg/m2/per week for the first month plus 2 infusions the first week of the following 2 consecutive months.
Efficacy and Safety of RituximabIn the randomised double blind phase III clinical trial,20 in the primary outcome it was observed that the survival of treatment of the group treated with RTX (n=28) at 12 months was higher than that of conventional treatment (n=29): glucocorticoids, azatioprine or cyclophosphamide or plasmaheresis, in patients with severe and active cryoglobulinaemic vasculitis (with skin ulcers, active glomerulonephritis, or refractory peripheral neuropathy or with a worsening of these conditions (64.3% vs 3.5%; P<.001). One of the secondary outcomes of the study tried to analyse the efficacy of treatment with RTX for each single case. The presence or non presence of neuropathy in patients with cryoglobulinaemic vasculitis had no impact on the drug's retention rate (hazard ratio 95% CI=4.05 [.91–18.03], P=.066). Neither did the presence or non presence of the other organic manifestations considered for the patient selection influence the retention rate of treatment with RTX: skin ulcers and glomerulonephritis. No significant differences were found for any level of improvement of the neuropathy after one month and after 2 months of treatment in the neuropathy group treated with RTX (n=16) compared with the conventional treatment group (n=17) (P=.156 and P=.375). Neuropathy responded to RTX after 2 months in 12 of the patients, after 6 months in 11 patients, after 12 months in 7 patients and after 24 months in 8 patients, concluding a possible lack of response to RTX after 6 months. Furthermore, the rate of serious adverse effects (pneumonia, cardiovascular events and liver failure) was observed to be lower in the group treated with RTX compared with those receiving conventional treatment (.12 vs .48). In the RTX group there were 2 deaths and in the group of conventional treatment there was one. There were no significant differences found in serious infections between both groups, but it should be pinpointed that 3 out of the 4 patients who had suffered from them had received high doses of glucocorticoids.
In the retrospective study carried out by Quartuccio et al.21 with a mean observation of 72.6 (20.4) months efficacy and safety were assessed long term in patients treated with RTX from the beginning, or who had changed to RTX during the De vita et al. clinical trial and if they needed retreatment with RTX this was only due to a worsening of their symptoms worsened (n=30); not included in the study were 3 patients in maintenance therapy with RTX, 5 patients who died, 2 who had reactions to the drug infusion and 11 who were without follow-up after the termination of the clinical trial. In this study 43.3% of the patients did not require retreatment, with a mean follow-up of 62.4 months. Out of the 17 patients who did require retreatment, 58.8% required only one cycle and 41.2% more than one cycle. The mean time up until retreatment to the initial dose was 22.3 (12.1) months. 85% of the patients who require retreatment after 24 months were because of the worsening of their neuropathy, with this being the same cause for which treatment with RTX was initiated. 20% of the patients who required retreatment after 24 months did so due to neuropathy. A mean of (± SD) RTX retention for neuropathy was observed at 6.7 (.4) years, somewhat lower than for nephritis (7.9 [.4]). During follow-up time after the clinical trial of De Vita et al. there were 9 adverse effects with RTX (recurrent infections, chronic hypogammaglobulinaemia, cardiovascular events) and 6 deaths.
In one multicentre retrospective study of 6 months follow-up,19 87 patients with mixed active cryoglobulaemic vasculitis were studied (92% associated with hepatitis C, 6% essential and 2% Sjogren syndrome), of whom 69 presented with peripheral neuropathy. In 20 of the 87 patients the main RTX indication was neuropathic involvement. Complete remission of neuropathy was achieved in 44% of patients and partial remission in 26% after 6 months of treatment. There was a statistically significant improvement of paresthesias (P<.0001). In the 87 patients treated 18 suffered from adverse effects, only 3 with serious adverse effects which were pneumonia, gangrene and serum sickness.
In a prospective cohort study of 13 patients with type II cryoglobulaemic vasculitis refractory to conventional treatment (12 of them associated with hepatitis C, 4 of them having being treated with interferon), a follow-up period of 12 months after initiation of treatment with RTX was made. A significant improvement was observed on the “clinical neuropathy disability score (CNDS)” scale (P<.001), electromyogram (P<.002) and paresthesias (P<.0001). No adverse effects were described during the follow-up period.
In a series of 6 cases with eosinophilic granulomatosis with active polyangitis in the form of severe pulmonary involvement and/or progressive peripheral neuropathy,18 5 of them (4 with positive neutrophil anticytoplasmic antibodies and one negative) were treated with RTX due to the reactivation of the neuropathy or to its remission. They presented with a mean of 10 months follow-up with assessment at 3 and at 6 months. Complete remission with RTX was obtained in 40% after 3 months and in 60% after 6 months, and partial remission was obtained in 20% after 6 months. Three patients presented with adverse effects during follow-up: reaction to the infusion with bronchospasm, and 2 patients had purulent bronchitis resolved with antibiotics.
DiscussionDespite the general efficacy demonstrated by RTX in vasculitis and systemic autoimmune diseases, there is very little information on the efficacy of this treatment specifically for vasculitic neuropathy.
Although clinical trials exist in which the efficacy of RTX has been demonstrated for ANCA-associated systemic vasculitis, there are no clinical trials in which neuropathy is specifically treated. In this sense, RTX is approved for inducing remission in granulomatosis with polyangitis and microscopic polyangitis, as was observed in the RITUXVAS and RAVE trials where it was at least as effective as cyclophosphamide.22,23 The efficacy of RTX for remission maintenance in granulomatosis with polyangitis and microscopic polyangitis was also recently confirmed in the prospective, randomised and controlled MAINRITSAN24 trial. Notwithstanding, in our review we found only one series of ANCA-associated cases (eosinophilic granulomatosis with polyangitis) which specifically included efficacy of RTX in vasculitic neuropathy where 80% of patients achieved remission after 6 months.18
The other studies which were finally selected for this review deal with the vasculitic neuropathy in the context of cryoglobulinaemic vasculitis. The findings from the randomised double-blind phase II clinical trial of De Vita et al.20 show the superiority of RTX in monotherapy compared with conventional therapy with glucocorticoids, azatioprine, ciclophosphamide or plasmapheris for treatment of severe cryoglobulinaemia as a form of greater treatment survival. In the multivariate analysis the presence or absence of neuropathy did not change this superiority. However, there were no significant differences for any degree of improvement of the neuropathy after one month and after 2 months of the treatment between the RTX group (n=16) and the group with conventional treatment (n=17) (P=.156 and P=.375). Caution should therefore be used when interpreting the specific efficacy in vasculitic neuropathy extracted from this study. However, the data of this comparison at 12 months follow-up are not available, and this was the time fixed for primary outcome, which could account for the absence of significant differences in this regard. In the long-term follow-up study of the previous clinical trial21 it was observed that the efficacy of RTX was considerably maintained over time and with a mean survival of RTX for neuropathy of 6.7 (.4) years, including patients who required retreatment for the same. Moreover, in the other 2 studies selected the efficacy of RTX was demonstrated in the form of complete or partial remission in the majority of patients with neuropathy and improvement in paresthesias, in scales and in objective data such as electromyographical parameters.17,19 It should be considered that except in the clinical trial, the rest of the data are not comparable with another treatment group and therefore the superiority of RTX cannot be established for vasculitic neuropathy in the context of cryoglobulinaemia, but its efficacy can, although we should take into account that RTX was effective in patients refractory to other conventional therapies including cyclophosphamide.
The majority of patients with mixed cryoglobulinaemia suffer from infection by the hepatitis C virus and this was also the case in the patients included in the studies which we selected. The cryoglobulinaemia may also be induced by the hepatitis B virus or associated with autoimmune or lymph proliferative disorders and on rare occasions, this may be essential.25,26 The current approach is that in patients with progressive or serious disease, apart from treating the viral infection (unless otherwise contraindicated), immunosuppressive treatment should also be administered: glucocorticoids with RTX or cyclophosphamide and in some patients plasmapheresis.15,27,28 In some cases of hepatitis B RTX could also be used, but always concomitant with antiviral therapy. Severe symptoms associated with cryoglobulinaemia where immunosuppressive treatment is recommended would include rapidly progressive neuropathy.
The safety profile of RTX was assessed in all of the studies reviewed. Adverse effects were described in all of them, with some being severe. However, only a few comparative data with conventional therapy were available. These showed that RTX presented a better safety profile in the form of a lower rate of severe adverse effects, which is in keeping with other studies reported in the literature. There were also several deaths in the studies reviewed, although they cannot be directly attributed to the RTX. In one observational study of 120 patients with granulomatosis with polyangitis and microscopic polyangitis, the patients simultaneously treated with RTX and cyclo phosphamide had similar remission rates to those who received RTX as monotherapy but with higher mortality.29 Among the adverse effects the following stand out: infections, hypogammaglobulinaemia, reaction to infusion and ischaemic cardiopathy. We should also highlight that in one of the studies neuropathy was found to be a secondary effect to treatment with RTX.
In the majority of studies analysed we described the initiation of treatment with RTX after failure with conventional treatment, but it has also been shown in the study by Ferri et al. that 15% of patients received RTX as first line treatment and in one patient eosinophilic granulomatosis with polyangitis, complete remission was achieved after 6 months.
Limitations of this review are firstly the low number of studies to meet the inclusion criteria of our review. Secondly, that the majority of these studies are observational, with only one clinical trial. Thirdly, the majority of the evidence is only related to one disease (essential mixed cryoglobulinaemia).
To conclude, we may say that there is little evidence of the efficacy of RTX for vasculitic neuropathy. It is probably effective for vasculitic neuropathy in the context of mixed cryoglobulinaemia and possibly superior to conventional treatment with a better safety profile. We did not find any data with which to extract conclusions regarding its efficacy in either isolated vasculitic neuropathy or that associated with systemic vasculitis and connective pathologies. We believe that having demonstrated its efficacy for rheumatoid arthritis and ANCA-associated vasculitis of RTX studies should be conducted to specifically address its efficacy in neuropathy to facilitate the best therapeutic option in cases where vasculitic neuropathy is the predominant symptom of a systemic clinical condition.
Conflict of InterestsThe authors have no conflict of interests to declare.
Spanish Society of Rheumatology (SSR).
Please cite this article as: Mena-Vázquez N, Cabezudo García P, Fuego Varela C, Manrique-Arija S, Fernandez-Nebro A. Eficacia y seguridad de rituximab en neuropatía vasculítica: revisión sistemática. Reumatol Clin. 2019;15:173–178.