Antagonists of tumour necrosis factor-alpha (ATNF) are used for the treatment of multiple diseases such as psoriatic arthritis, Crohn's disease, ankylosing spondylitis and juvenile idiopathic arthritis, usually, when they are refractory to first-line treatment.1 The use of ATNF has been associated with the induction of autoimmune diseases such as systemic lupus erythematosus-like disease, vasculitis, sarcoidosis-like diseases and, recently, acute granulomatous tubulointerstitial nephritis. We report a case of acute nongranulomatous tubulointerstitial nephritis in an HLA-B27-positive patient with axial spondyloarthritis and Crohn's disease being treated with adalimumab.
Los antagonistas del factor de necrosis tumoral-alfa (ATNF) se utilizan en el tratamiento de múltiples enfermedades, como artritis psoriásica, enfermedad de Crohn, espondilitis anquilosante, artritis idiopática juvenil, generalmente, cuando son refractarias al tratamiento de primera línea.1 La utilización de los ATNF se ha asociado con la inducción de enfermedades autoinmunes, como lupus eritematoso sistémico-like, vasculitis, sarcoidosis-like y, recientemente, nefritis túbulo-intersticial aguda granulomatosa. Describimos un caso de nefritis túbulo-intersticial aguda no granulomatosa en un paciente con espondiloartritis axial HLA-B27 positiva y enfermedad de Crohn en tratamiento con adalimumab.
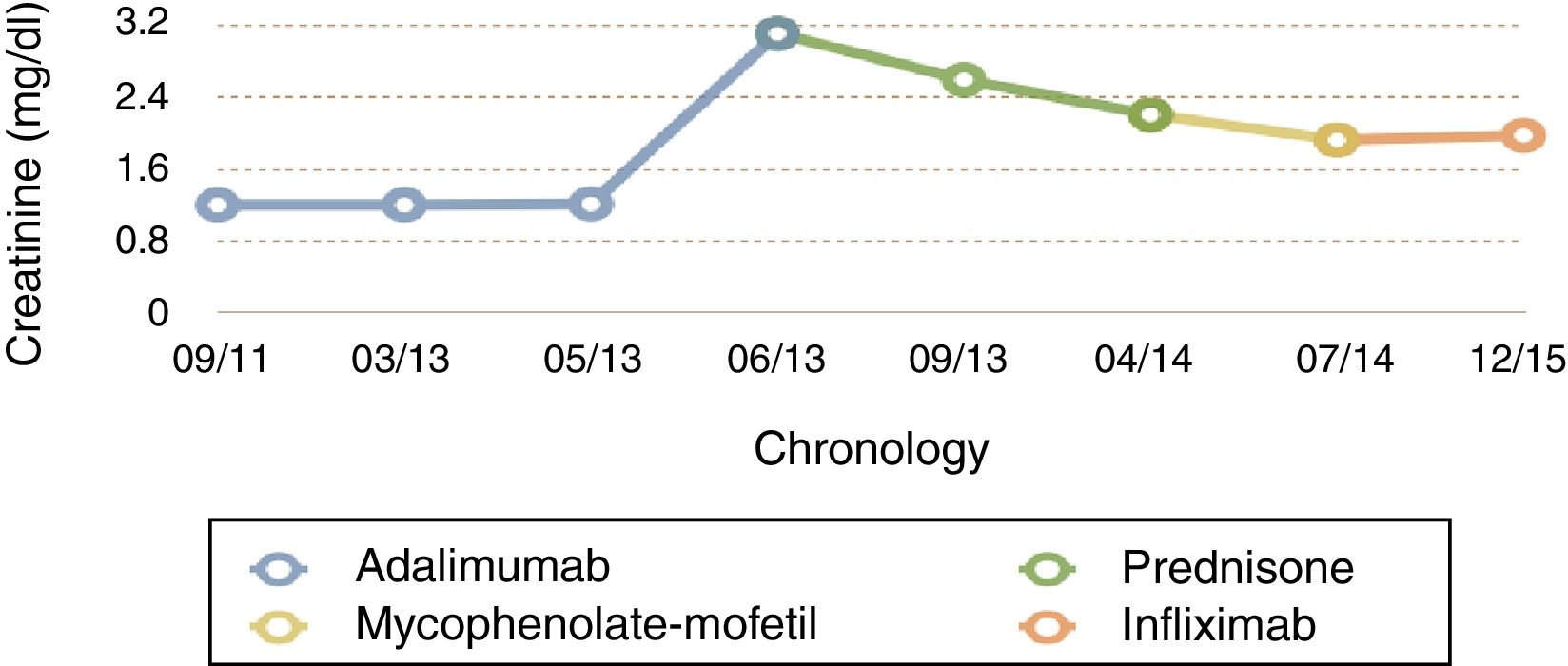
A patient aged 55 was referred from another hospital due to raised creatinine levels (3mg/dl) in a routine test (1.2mg/dl one year previously), but with no other symptoms.
The patient had a personal history of high blood pressure, type II diabetes mellitus controlled with oral anti-diabetic drugs, Crohn's disease with transverse colon colectomy, axial HLA-B27 positive spondyloarthritis, in follow-up by the rheumatology unit and had been in treatment with a TNF inhibitor, adalimumab, at a subcutaneous dose of 40mg every 2 weeks for 27 months and with tramadol. He had also previously sporadically used celecoxib.
A physical examination highlighted blood pressure of 166/86mmHg, cardiopulmonary and abdominal auscultation were normal, with no oedemas or arthritis or skin lesions.
Laboratory tests on admittance showed a red blood count of 9.7g/dl with the remainder of the blood count normal and with no eosinophilia. Urea was at 68mg/dl (N: 20–50) and creatinine at 3.1mg/dl (N: .64–1.27). Liver function, creatine kinase, lactate dehydrogenase, cholesterol, triglycerides, ionogram, proteinogram and immunoglobulins tested normal. Antinuclear antibodies, anti-neutrophil cytoplasmic antibodies and anticardiolipins (IgM and IgG) tested negative. Ferrokinetic, thyrotropin, parathyroid hormone, carcinoembryonic antigen, Ca19.9, prostate-specific antigen and alpha-fetoprotein studies were normal. The urine system was strictly normal, with a sediment with 4 red blood cells per field and 4 white blood cells per field and a protein/creatinine ratio of 0.29, sodium in urine of 72.8mmol/l, with no presence of eosinophils. A chest X-ray did not show any cardiomegaly or pulmonary infiltrates; the echocardiogram showed well preserved ejection fraction, the back of the eye test showed slight diabetic retinopathy and the kidney scan showed normal corticomedullary size and differentiation and with no signs of obstructive uropathy.
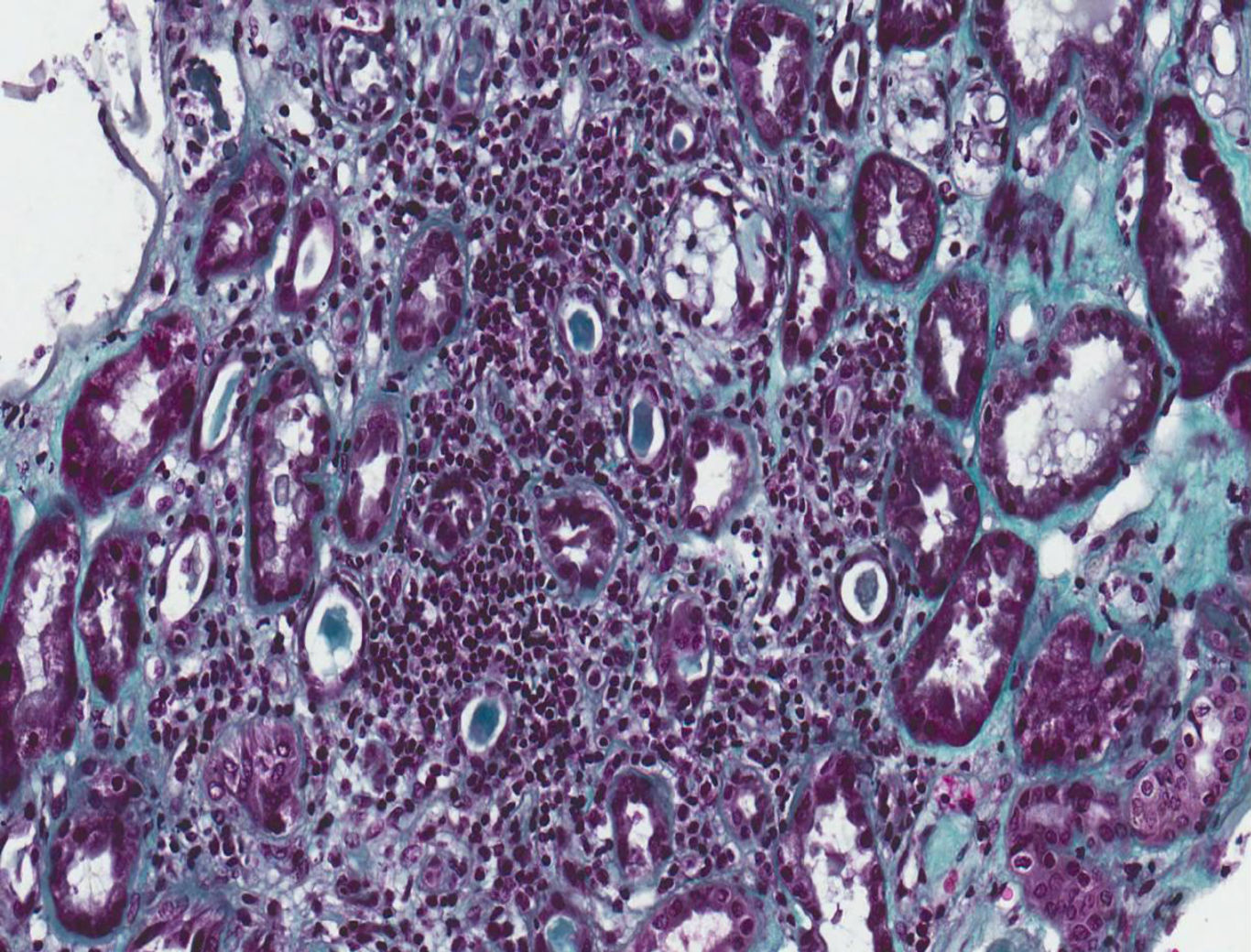
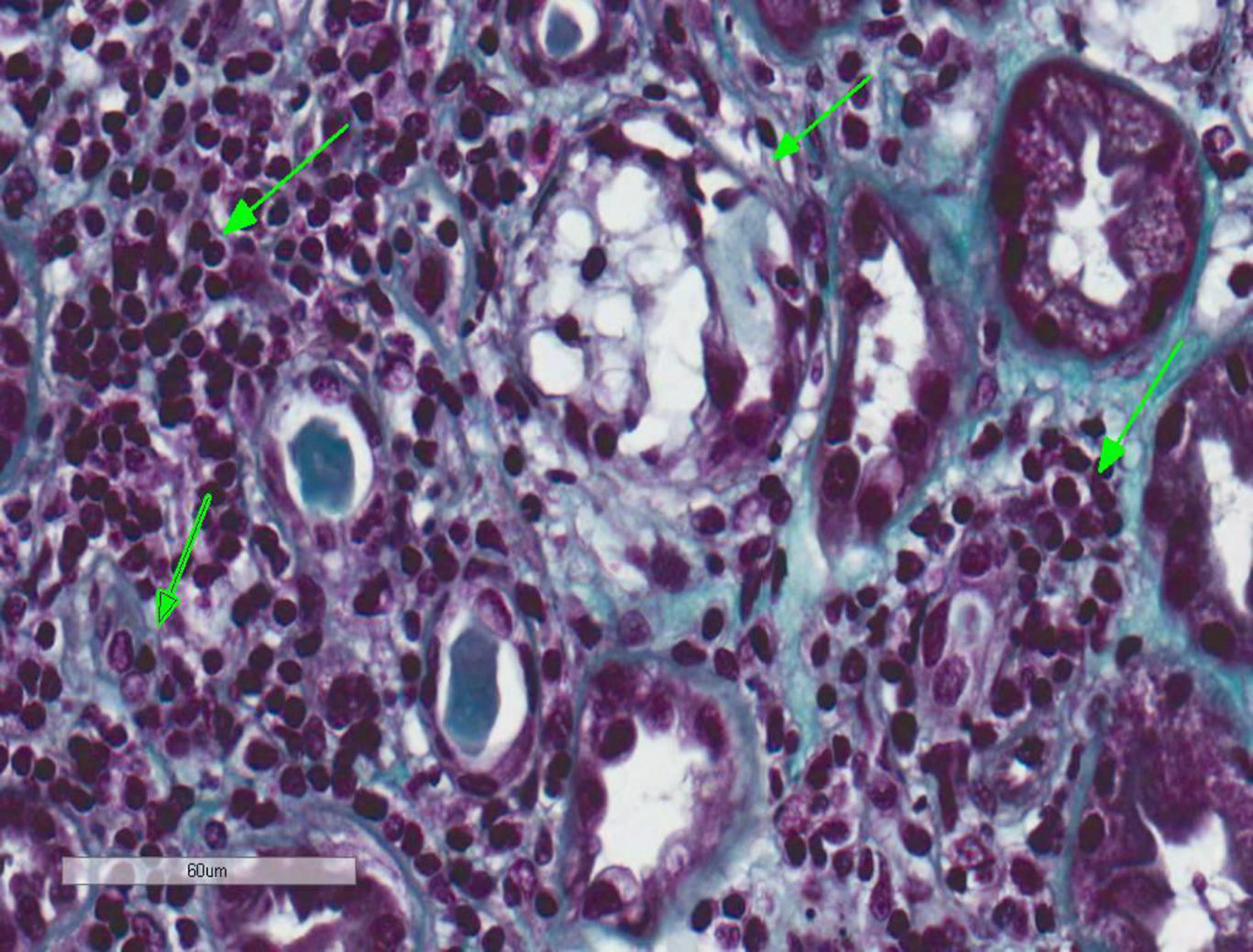
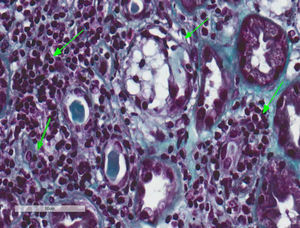
A kidney biopsy was performed where 17 clusters were obtained, with fibroedema in the interstitium and antinflammatory infiltrate of lymphocytes inside the tubule (tubulitis), with partial destruction of the latter, and occasional infiltration of the tubular epithelium. The tubules presented with cytoplasmatic vacuolisation and desquamation of the intraluminal epithelium with some lymphocytes on the inside, few tubules with presence of pin-type cells with hyperchromatic nuclei, enhanced in size and with flat epithelium. In immunofluorescence slight granular deposits of IgM and C3 were detected and no relevant changes were observed in the electronic microscopy. This was all compatible with acute tubulointerstitial nephritis (TIN) and secondary acute tubular necrosis, with no presence of granulomas or diabetic nephropathy (Figs. 1 and 2).
Tubulointerstitial nephritis and a mild case of secondary acute tubular necrosis. Kidney biopsy. Lymphocytary inflammatory infiltration which mainly affects the insterstitium and lymphocyte permeation in the tubular epithelium with partial destruction of it, compatible with TIN. Few tubules present regenerative changes with flat epithelium and cytoplasmatic vacuolisation with nuclei enhanced in size and hyperchromatic and with intraluminal epithelial desquamation (secondary acute tubular necrosis) (Masson's trichrome, 40×).
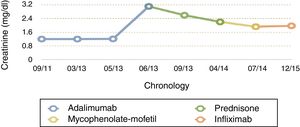
During his stay, the patient remained clinically asymptomatic with creatinine levels between 2.8 and 3.1mg/dl, a protein/creatinine ratio between .16 and .29 and isolated red blood cells in urine sediment. As a result of all these factors adalimumab was discontinued, in case this was the cause of the non-granulomatous idiosyncratic TIN, and treatment was initiated with corticoids at a dose of .5mg/kg/day, with stable creatinine levels at 2.6mg/dl after 11 weeks. Further studies were made with a chest CT scan which showed no granulomas or lymphadenopathies; the Mantoux test was negative, baciloscopes were negative; mycobacteria and culture BAAR was negative; converting enzyme (ACE) inhibitor negative and the lab did not consider performing mycobacterium tuberculosis by PCR.
For 10 months the administration of corticoids was gradually reduced until it was withdrawn. After this, with creatinine at 2.2mg/dl, and with no clinical features of spondyloarthropathy or Crohn's disease, we decided to start treatment with mycophenolate-mofetil (MMF), on suspicion of corticoid refractory TIN. After 3 months of MMF, creatinine was 1.92mg/dl and, following a severe outbreak of Crohn's disease with a 10kg weight loss and extensive diarrheal stools the MMF was suspended and intravenous infliximab was administered at a dose of 5mg/kg at 0.2 and at 6 weeks (and subsequently, every 8 weeks). After 2 months of treatment the patient was found to be asymptomatic with stable creatinine levels of 1.96mg/dl and urea of 44mg/dl, with no proteinuria (Fig. 3).
At present, and after 18 months of treatment with infliximab, there has been no impairment of kidney function (last creatinine test levels at 1.7mg/dl) and the patient's Crohn's disease and sponyloarthritis has been kept completely under control.
DiscussionTNF-α is a proinflmmatory cytokine which is mainly synthesised in monocytes/macrophages and T lymphocytes, promoting inflammation by direct cytotoxic effect and indirect effects such as the regulation of the production of other proinflammatory cytokines (IL-1, IL-6 and IL-8), liberation of free radicals of oxygen and nitrogen, metalloprotoneinases, chemokines and antiangiogenic factors. According to the standard proinflammatory model, when an immune response fails in the regulation of TNF-α, innate cellular immunity is activated and chronic inflammatory response leads to tissue damage. Neutralisation of the TNF-α activity with drugs such as the ATNF, leads to deactivation of the proinflammatory cytokines, reduction of the inflammatory cells and the possibility of angiogenesis, changes in the chemokines and in vascular permeability. TNF inhibitor drugs began to be used at the end of the 1990s to treat rheumatoid arthritis and Crohn's disease. At present, there are 5 TNF antagonists, 4 monoclonal antibodies (infliximab, adalimumab, golimumab and certolizumab) and a fusion protein of the TNF soluble receptor (etanercept), which are used for many different diseases, including rheumatoid arthritis, ankylosing spondylitis,1 juvenile idiopathic arthritis, ulcerative colitis, Crohn's disease, psoriasis and uveitis.2,3
The most common side effects of TNF inhibitors are standard, opportunist and/or granulomatous infections, including histoplasmosis, listeriosis and extrapulmonary tuberculosis; malignant diseases such as Non-Hodgkin's lymphoma and skin growths,4 exacerbated heart failure, cytopenias, neutrological demyelinating disease and immunogenicity.5 However, regarding the kidneys, adalimumab has been associated with non-filiated kidney failure, haematuria and proteinuria.6–9 Furthermore, secondary nephrotic syndrome has been associated with membranous glomerulonephritis after 10 months of treatment, referred to by Gupta et al.,10 and class III lupus nephritis 15 months after treatment was started, commented upon by Stokes et al.11,12 Justo Ávila et al. described that the granulomatous diseases may induce TIN in patients being treated with adalimumab.13
TIN is an acute inflammation of the tubule and interstitium. Its most common cause is the use of drugs such as antibiotics, non-steroid anti-inflammatory drugs and diuretics, although it many also present in the context of metabolic and immunologic diseases, neoplasms or infections, similarly to other interstitial disorders.14 In the case of our patient, prior to the kidney failure, the selective inhibitor of the cyclo-oxygenase-2 (celecoxib) which he took sporadically had been suspended weeks before treatment started with adalimumab for 21 months (a similar interval to those published by Gupta et al., Stokes et al., Justo Ávila et al. and Korsten et al.). This leads us to the belief that the celecoxib was not the cause of the TIN but that adalimumab was responsible for it.
Justo Ávila et al. describe that granulomatous diseases may induce TIN, and present a patient with tuberculosis after 24 months of administration of adalimumab, but the presence of granulomas in the biopsy, lead us to thinking that the TIN is due to the tuberculosis rather than the drug.13 Korsten et al. also describe a sarcoidosis after 18 months of adalimumab with granulomatous TIN in the kidney biopsy.15 These authors suggest that involvement of the kidney is secondary to adalimumab, but the pathogenesis they refer to is a scarcoid type granulomatous inflammatory process.
In our case, having ruled out a granulomatous disease and the posterior course of the disease makes us think that the TIN could have been secondary to the adalimumab, and that in the previously described cases, in addition to the granulomatous disease causing kidney involvement, adalimumab played a major role, as postulated by previous authors.
Ethical DisclosureProtection of people and animalsThe authors declare that for this research no experiments have been carried out on humans or animals.
Confidentiality of dataThe authors declare that they have adhered to the protocols of their centre of work on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of InterestThe authors have no conflict of interests to declare.
I would like to thank both the Rheumatology service and the Nephrology and Pathological Anatomy services for their collaboration and effort, since without them the patient would not have been able to be appropriately treated and their quality of life improved.
Please cite this article as: Castro Corredor D, Sánchez de la Nieta MD, de Lara Simón IM. Nefritis túbulo-intersticial aguda en paciente con espondiloartritis axial HLA-B27 en tratamiento con adalimumab. Reumatol Clin. 2019;15:179–181.