Evaluate the effectiveness, cost and safety of rituximab in patients with rheumatoid arthritis (RA) depending on the dose used.
Material and methodsRetrospective observational study conducted on 52 patients with RA treated with at least one dose of rituximab for 135.3 patient-years were included. Three treatment groups were obtained: (G1) First course and following two 1g infusions separated by 15 days; (G2) First course 2 infusions of 1g followed by 2 infusions of 500mg; (G3) First course and followed by 2 infusions of 500mg separated by 15 days. Re-treatments were administered on-demand according to the clinical activity. The retention time (Log-Rank), retreats and adverse events rates (incidence rate ratio) and treatment costs per patient-month of rituximab were analyzed by groups.
ResultsGroup 2 showed a better cost-effectiveness ratio than group 1, as it was associated with a longer retention of rituximab (mean [95% CI] 65.7 [60.8–70.7] months vs 33.5 [22.7–44.3]; P<.001) and a lower rate of severe adverse events with only a slight increase in the rate of retreatment (courses/patient-year [95% CI] 1.66 [1.39–1.93] vs 1.01 [0.69–1.34]; P=.005), and in the costs (median/patient-month, €484.89 vs €473.45). Although group 3 was €41.20/patient-month cheaper than group 2, it was associated with a higher rate of re-treatments and shorter retention of rituximab (P<.001).
ConclusionsThe use of full-dose rituximab at onset, followed by reduced doses in successive courses administered on-demand retreatment may be the most cost-effective option.
Evaluar la efectividad, seguridad y coste de rituximab en pacientes con artritis reumatoide (AR) dependiendo de la dosis utilizada.
Material y métodosEstudio observacional retrospectivo. Se incluyó a 52 pacientes con AR tratados al menos con una dosis de rituximab durante 135,3 pacientes-año. Se obtuvieron 3 grupos de tratamiento: G1, primer curso y siguientes de 2 infusiones de 1g separadas 15 días; G2, primer curso de 2 infusiones de 1g seguido por cursos de 2 infusiones de 500mg, y G3, primer curso y siguientes de 2 infusiones de 500mg separadas por 15 días. Los retratamientos fueron a demanda según la clínica. Se analizaron por grupos: el tiempo retención (Log-Rank), las tasas de retratamientos y de eventos adversos (razón de tasas de incidencia) y los costes del tratamiento por paciente-mes de rituximab.
ResultadosEl grupo 2 mostró una mejor relación coste-efectividad que el grupo 1 ya que se asoció a una mayor retención de rituximab (media [IC del 95%] 65,7 [60,8-70,7] meses vs 33,5 [22,7-44,3]; p<0,001) y una menor tasa de eventos adversos graves, con solo un ligero incremento de la tasa de retratamientos (cursos/paciente-año [IC del 95%] 1,66 [1,39-1,93] vs 1,01 [0,69.-1,34]; p=0,005) y del coste (mediana/paciente-mes, 484,89 € vs 473,45 €). Aunque el grupo 3 fue 41,20 €/paciente-mes más económico que el grupo 2, se asoció a una mayor tasa de retratamientos y una menor retención de rituximab (p<0,001).
ConclusionesEl uso de rituximab a dosis completa al inicio seguido de dosis reducida en los sucesivos cursos administrados a demanda parece la opción más coste-efectiva.
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic synovial inflammation, joint destruction and functional disability.1
Rituximab is a chimeric, anti-CD20, B-cell-depleting monoclonal antibody composed of a human and a murine portion. It has been approved for use in combination with methotrexate in patients with moderately or highly active RA who have not responded satisfactorily to anti-tumor necrosis factor (anti-TNF) therapy.1 A number of studies have demonstrated that changing to rituximab rather than to another anti-TNF agent may prove to be more effective.2–4Although biological treatments represent an important advance in the control of RA, their potential toxicity and high cost should be taken into account. Given both their elevated price and the costs of administration, monitoring, prophylaxis and treatment of the toxicity, they are cost-effective only in certain situations.5–7 Although the price per dose of rituximab is higher than that of the anti-TNF agents, as the treatment with the former requires fewer courses, in the end, it is the less expensive of the two.
The authorized dose of rituximab is 2 infusions of 1000mg separated by 15 days for each treatment course, and repeat treatments are administered every 6 months.8 However, at the present time, patient management is being generalized by means of “tight control” and “treat to target” strategies. These strategies require frequent adjustments of the treatment intensity and have opened the door to modifications of the doses and regimens recommended in the specifications, depending on the clinical status of the patient. Moreover, the authorized rituximab doses have been found to be only slightly superior in the prevention of radiation damage, but there was no clear clinical superiority in the major phase 3 trials with rituximab.9–16As a consequence of these approaches, studies have been conducted in the attempt to determine whether the same therapeutic benefit is obtained in patients receiving rituximab at a dose of 500mg, rather than the standard dose of 1g.14–16 The results suggest that, in some patients, the reduced dose can exhibit the same efficacy, as was observed in the DANCER study,9 SERENE trial,10 TAME study,11 MIRROR trial12 and IMAGE trial.13
On the other hand, in the clinical trials that evaluate the efficacy of rituximab for the treatment of RA, it has been observed that the most frequent adverse events are associated with reactions to infusion of the drug and the rate of infusion. This suggests that a reduction in the doses might also be accompanied by a lower incidence of adverse events.
Finally, it is not known whether or not a decrease in the dose of rituximab is associated with a higher frequency of retreatment when it is administered at the discretion of the treating physician. This datum is relevant as regards the efficacy of this drug, as a reduction in the dose could be accompanied by a reduced effectiveness and an even higher cost, should retreatment be required more frequently.
In short, clinical practice studies that evaluate the true efficacy of the use of rituximab in regimens of this type would be necessary.
Our objective was to investigate whether there were differences in efficacy in terms of effectiveness (measured by the treatment retention time and retreatment rates), safety and final cost among patients treated with different regimens and doses of rituximab.
Patients and MethodsDesign and Study SettingWe performed a retrospective observational study based on the review of the medical records of RA patients treated with rituximab at the Hospital Regional Universitario de Málaga, Spain (a tertiary care center with a reference population of 628,912 inhabitants). The study was reviewed and approved by the hospital's clinical research ethics committee.
PatientsAll the patients treated with rituximab between July 2006 and December 2012 were included in the study. The eligibility criteria were age ≥18 years, RA according to the 1987 American Rheumatism Association criteria,17 and having been treated with at least 1 rituximab infusion. Patients who had been followed for less than 3 months after the first dose of rituximab were excluded.
Measurements and VariablesMain outcome measureThe main outcome of treatment effectiveness was measured by the rituximab treatment retention time in patient-years (including repeat courses).
Secondary Outcome Measures1) Rates of retreatment adjusted for exposure time and incidence rate ratio (IRR) in the 3 study groups; 2) treatment safety, based on the type, severity and incidence of serious and minor events throughout the entire study period, according to patient group; and 3) costs of rituximab per patient-month associated with each treatment group, estimated by cost minimization from the care provider perspective.
Safety VariablesThe presence or absence of serious and minor adverse events and their descriptions were recorded for all the patients at each visit. The safety profile was evaluated, first, by calculating the incidence rates of total and serious adverse events. This was done by dividing the total number of adverse events by the follow-up time of each patient in years (events/patient-year). Subsequently, the incidence rates in each group were calculated in the same way and compared using the IRR. The same operation was carried out taking only the serious adverse events.
Cost CalculationThe calculation of the costs associated with rituximab in each group was based on the purchase price of the drug corresponding to the latest tender of the procurement platform of the province of Málaga. The costs derived from its administration in the day hospital or the infusion systems, staff, etc., were not taken into account. This gave us a mean price for rituximab of €1199.61 per 500-mg vial for infusion throughout the years of the study period.
To analyze the overall costs, first the real total cost of the treatment was calculated by adding up the partial costs in each group throughout the entire study. This result was then adjusted for the follow-up time of each patient, which gave us the cost in euros per patient-month. These results were collected by group, the mean±standard deviation (SD) and median were obtained for each, and the findings were compared.
Remaining VariablesDates. Dates of the diagnosis of RA, of the protocol (data collection from the patient's medical record), of the first course of rituximab, of the first infusion of the first course, of the last course of rituximab and of the first infusion of the last course. The dates of other events were also recorded: date of discontinuation of rituximab (desired effect achieved) or of the last visit if the patient was still being actively treated, date of discontinuation of rituximab (desired effect not achieved) and date of the decision to change the treatment because of: a) disease reactivation (inefficacy); b) development of adverse event; or c) decision on the part of the physician or patient.
Courses and doses. 1000-mg course (2 infusions of 1000mg separated by a 2-week interval) and 500-mg course (2 infusions of 500mg separated by a 2-week interval). Rituximab dose in the last course: grams of rituximab administered in the last course.
Laboratory tests. Rheumatoid factor, measured in U/mL, where titers above the upper cutoff point used in our laboratory (>20U/mL) were considered to be elevated, and anti-cyclic citrullinated peptide antibody, measured in U/mL, where titers over 10U/mL were considered to be positive.
Costs. Price per vial: mean cost per 500-mg vial in our hospital, €1199.61. Expected monthly cost: monthly cost in euros (€) of rituximab treatment administered in 2 courses a year (every 6 months), each consisting of 2 infusions of 1000mg separated by a 15-day interval (€799.74). Total expected cost: result of multiplying the expected monthly cost by the total of patient-months. Real total cost: final cost in euros of rituximab administered to the patients included in the study. Serious adverse effect: an effect that results in one or more of the following: death, a threat to life, a congenital malformation or birth defect, persistent significant disability, or need for or prolongation of hospitalization.
Treatment Protocol and GroupsThe RA patients being treated with biological agents in our hospital are evaluated in a specific day hospital unit. For this study, the evaluation consisted of the systematic, prospective collection of data on effectiveness and safety using a predesigned form. The drug doses were decided by the patients’ physicians on the basis of the clinical context; they also decided on the administration of full or reduced doses in each case.
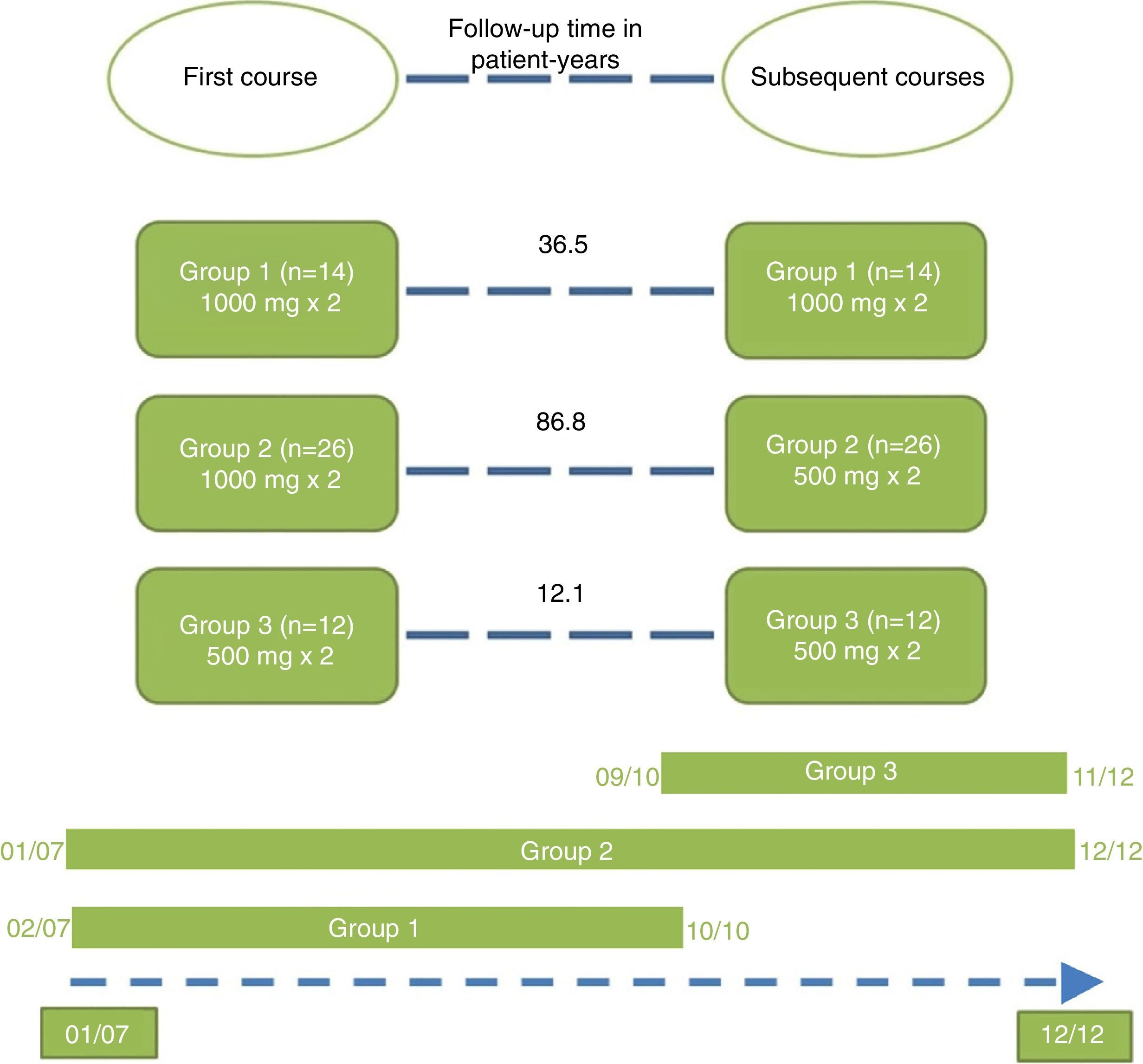
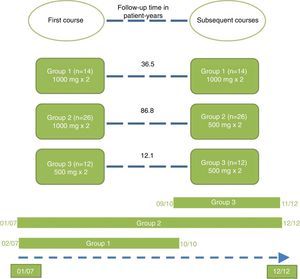
Repeated courses were administered at the discretion of the treating physician, once 6 months had elapsed since the date of the first infusion of the first course. The patients were distributed into 3 treatment groups according to the doses of rituximab they had received. Group 1 (standard dose): first course and successive courses consisting of 2 infusions of 1000mg separated by a 2-week interval. Group 2 (standard induction dose with reduced doses thereafter): first course consisting of 2 infusions of 1000mg separated by a 2-week interval, followed thereafter by courses consisting of 2 infusions of 500mg separated by a 2-week interval. Group 3 (all doses reduced): first course and successive courses consisting of 2 infusions of 500mg separated by a 2-week interval.
Statistical AnalysisDescriptive analysis was performed in the sample and inferential analysis in the different rituximab treatment groups, using ANOVA/Kruskal–Wallis for the continuous variables and the chi-square test for the categorical variables. Survival associated with rituximab was analyzed with Kaplan–Meier curves and the comparison between groups by the log-rank test. The number of repeated treatments adjusted for exposure time was evaluated using incidence rates (IR) and compared by means of IRR. Multivariate analysis was carried out with Cox regression.
ResultsFrom July 2006 to December 2012 (inclusive), 57 patients with RA were treated with at least 1 course of rituximab. Five patients were excluded because less than 3 months had elapsed since their first rituximab course when enrollment in the study was concluded. The final analysis included 52 patients with a total follow-up of 135.34 patient-years.
The patients were divided into 3 groups according to the doses of rituximab they received in the first course and thereafter (Fig. 1). Group 2 was the largest of the 3 and showed the longest treatment retention time.
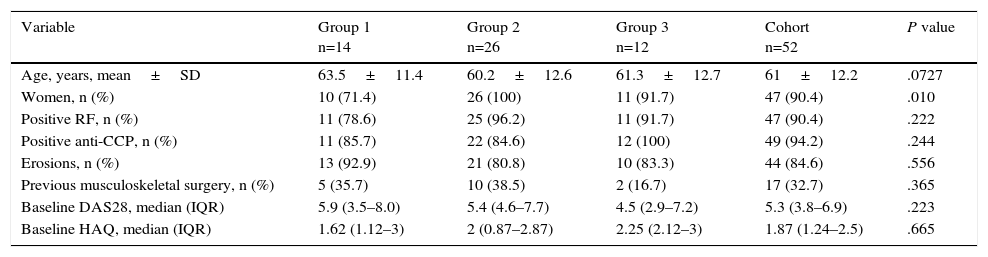
The baseline clinical characteristics of the patients are shown according to group in Table 1. The majority of the patients were women with a mean age of 61 years, were seropositive and had erosive disease. The proportion of women in group 1 was lower than in the other 2 groups. There were no significant differences between the 3 groups with respect to the median disease activity score in 28 joints (DAS28) at baseline. Three months after the first infusion, the median DAS28 (calculated from the available data) had decreased in all 3 groups (group 1=3.4; group 2=3.2; group 3=3.94), again without significant differences (P=.16).
Baseline Clinical Characteristics According to Treatment Group.
| Variable | Group 1 n=14 | Group 2 n=26 | Group 3 n=12 | Cohort n=52 | P value |
|---|---|---|---|---|---|
| Age, years, mean±SD | 63.5±11.4 | 60.2±12.6 | 61.3±12.7 | 61±12.2 | .0727 |
| Women, n (%) | 10 (71.4) | 26 (100) | 11 (91.7) | 47 (90.4) | .010 |
| Positive RF, n (%) | 11 (78.6) | 25 (96.2) | 11 (91.7) | 47 (90.4) | .222 |
| Positive anti-CCP, n (%) | 11 (85.7) | 22 (84.6) | 12 (100) | 49 (94.2) | .244 |
| Erosions, n (%) | 13 (92.9) | 21 (80.8) | 10 (83.3) | 44 (84.6) | .556 |
| Previous musculoskeletal surgery, n (%) | 5 (35.7) | 10 (38.5) | 2 (16.7) | 17 (32.7) | .365 |
| Baseline DAS28, median (IQR) | 5.9 (3.5–8.0) | 5.4 (4.6–7.7) | 4.5 (2.9–7.2) | 5.3 (3.8–6.9) | .223 |
| Baseline HAQ, median (IQR) | 1.62 (1.12–3) | 2 (0.87–2.87) | 2.25 (2.12–3) | 1.87 (1.24–2.5) | .665 |
CCP, cyclic citrullinated peptide; DAS, disease activity score in 28 joints; HAQ, health assessment questionnaire; IQR, interquartile range; RF, rheumatoid factor; SD, standard deviation.
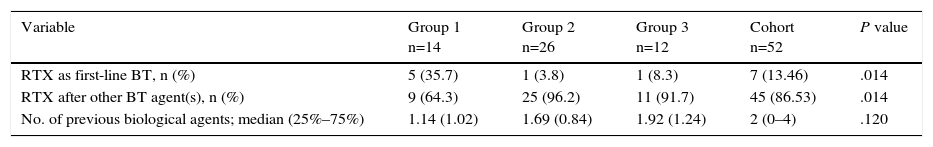
The majority of the patients began rituximab therapy after having received at least 1 anti-TNF agent (Table 2). The drugs most frequently administered with rituximab as concomitant treatment during the first course were methotrexate and corticosteroids.
Sequence of Rituximab Use and Previous Biological Agents According to Treatment Group.
| Variable | Group 1 n=14 | Group 2 n=26 | Group 3 n=12 | Cohort n=52 | P value |
|---|---|---|---|---|---|
| RTX as first-line BT, n (%) | 5 (35.7) | 1 (3.8) | 1 (8.3) | 7 (13.46) | .014 |
| RTX after other BT agent(s), n (%) | 9 (64.3) | 25 (96.2) | 11 (91.7) | 45 (86.53) | .014 |
| No. of previous biological agents; median (25%–75%) | 1.14 (1.02) | 1.69 (0.84) | 1.92 (1.24) | 2 (0–4) | .120 |
BT, biological treatment, RTX: rituximab.
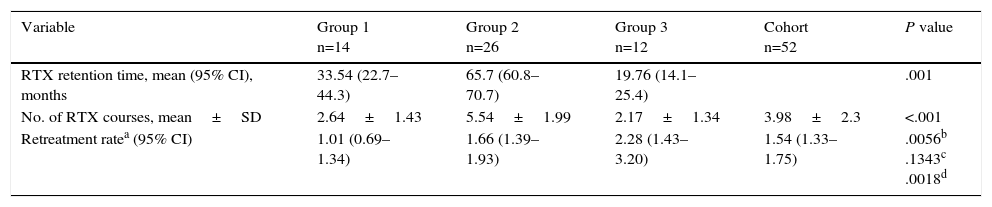
The study patients underwent a mean follow-up (±SD) of 31.2±18 months and received 1.54 courses of rituximab/patient-year (95% confidence interval 1.33–1.75). The patients receiving the reduced dose (groups 2 and 3) required a higher rate of repeated rituximab treatments then group 1 (standard dose), with differences that were statistically significant according to the IRR (Table 3). There were no significant differences between groups 2 and 3. According to multivariate analysis, no baseline variable had an influence on the rituximab treatment retention time.
Retention Time and Rate of Rituximab Retreatment According to Treatment Group.
| Variable | Group 1 n=14 | Group 2 n=26 | Group 3 n=12 | Cohort n=52 | P value |
|---|---|---|---|---|---|
| RTX retention time, mean (95% CI), months | 33.54 (22.7–44.3) | 65.7 (60.8–70.7) | 19.76 (14.1–25.4) | .001 | |
| No. of RTX courses, mean±SD | 2.64±1.43 | 5.54±1.99 | 2.17±1.34 | 3.98±2.3 | <.001 |
| Retreatment ratea (95% CI) | 1.01 (0.69–1.34) | 1.66 (1.39–1.93) | 2.28 (1.43–3.20) | 1.54 (1.33–1.75) | .0056b .1343c .0018d |
CI, confidence interval; IRR, incidence rate ratio; RTX, rituximab; SD, standard deviation.
The treatment retention time in group 2 was twice that of group 1 and up to 7 times longer than that of group 3 (Table 3).
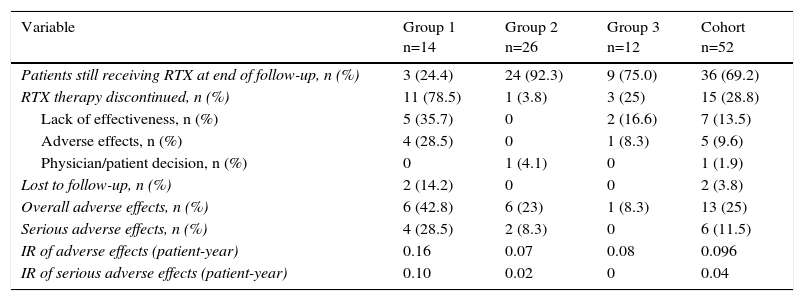
Reasons for Discontinuing Treatment and Adverse EffectsThe treatment was discontinued in 15 patients (28.8%), most often for the lack of effectiveness, followed by adverse effects (Table 4). At the end of follow-up, group 2 had the highest number of patients still being actively treated, followed by group 3 (Table 4).
Causes for Discontinuation and Adverse Effects of Rituximab According to Treatment Group.
| Variable | Group 1 n=14 | Group 2 n=26 | Group 3 n=12 | Cohort n=52 |
|---|---|---|---|---|
| Patients still receiving RTX at end of follow-up, n (%) | 3 (24.4) | 24 (92.3) | 9 (75.0) | 36 (69.2) |
| RTX therapy discontinued, n (%) | 11 (78.5) | 1 (3.8) | 3 (25) | 15 (28.8) |
| Lack of effectiveness, n (%) | 5 (35.7) | 0 | 2 (16.6) | 7 (13.5) |
| Adverse effects, n (%) | 4 (28.5) | 0 | 1 (8.3) | 5 (9.6) |
| Physician/patient decision, n (%) | 0 | 1 (4.1) | 0 | 1 (1.9) |
| Lost to follow-up, n (%) | 2 (14.2) | 0 | 0 | 2 (3.8) |
| Overall adverse effects, n (%) | 6 (42.8) | 6 (23) | 1 (8.3) | 13 (25) |
| Serious adverse effects, n (%) | 4 (28.5) | 2 (8.3) | 0 | 6 (11.5) |
| IR of adverse effects (patient-year) | 0.16 | 0.07 | 0.08 | 0.096 |
| IR of serious adverse effects (patient-year) | 0.10 | 0.02 | 0 | 0.04 |
IR, incidence rate; RTX, rituximab.
Group 1 had the highest number of dropouts, again, mainly due to lack of effectiveness, followed by adverse effects. Despite the fact that group 2 had the greatest number of patients and the longest exposure time, it was the group with the fewest dropouts. Group 3, like group 1, although to a lesser extent, also had more dropouts than group 2, and, here too, the reasons were lack of effectiveness or adverse effects.
The overall rate of adverse effects in the entire sample was 0.096 adverse effects/patient-year, whereas that rate of serious effects was 0.04 adverse effects/patient-year. With respect to differences between groups, group 1 had the highest overall rate of adverse effects and group 2 had the lowest. When only serious adverse effects were evaluated, the findings were the same, with group 1 having the highest rate and group 2, the lowest (Table 4).
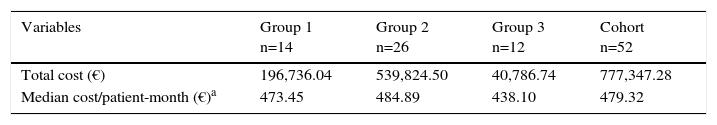
Costs of RituximabThe real overall cost of treatment in all the patients (n=52) was €777,347.28 (Table 5), whereas the total expected cost (if all the patients had been treated systematically every 6 months) was €1,298,841.74. This represented a theoretical savings for the hospital of €521,494.46 (€10,028.74/patient-year).
Costs in Euros per Treatment Group.
| Variables | Group 1 n=14 | Group 2 n=26 | Group 3 n=12 | Cohort n=52 |
|---|---|---|---|---|
| Total cost (€) | 196,736.04 | 539,824.50 | 40,786.74 | 777,347.28 |
| Median cost/patient-month (€)a | 473.45 | 484.89 | 438.10 | 479.32 |
With regard to the monthly cost per patient in each of the groups, in group 2, the median cost (€484.89/patient-month) was only €11.44 higher than that of group 1 (€473.45/patient-month) and €46.79 higher than that of group 3 (€438.10/patient-month). This means that the savings with group 3 (the least costly) with respect to group 2 (the most costly) reached €561.48/year, whereas that of group 1 represented a savings of only €137.28/year, despite its being the group in which full doses were always administered, according to the specifications.
Taking into account the cost-effectiveness ratio of rituximab in terms of the doses received, according to our findings, group 2 was the most efficient as it was associated with a greater treatment retention and a lower rate of serious adverse effects than group 1, with only slight increases in the rate of repeated treatments and the cost.
DiscussionThe economic impact of RA is largely due to the cost of treatment. A great part of this expense corresponds to biological therapy.18 For this reason, we consider that the choice of therapy should depend not only on its efficacy, but on its efficiency, as well. Another aspect that is equally important is the safety profile of the drug.
We carried out a retrospective observational study to evaluate the efficiency of reduced rituximab doses for the treatment of RA based on our clinical practice.
We chose this retreatment scheme because many patients in clinical practice remain stable for periods of over 6 months.19 Moreover, this approach makes it possible to reduce expenses and the number of treatment courses accumulated throughout their lives. At the present time, we have no conclusive data on the long-term safety of repeated courses, and there is no evidence of differences in terms of the safety between the fixed treatment scheme and treatment at the discretion of the treating physician.20
A number of factors influence the retention time of a drug, such as its effectiveness, its toxicity, the availability of other treatment alternatives, the severity of the disease and adherence to treatment.21 However, due to the fact that there are now different options for biological therapy in RA, a longer treatment retention time usually translates into its greater effectiveness and safety.
In our study, there were no differences in the baseline clinical characteristics of the 3 treatment groups, with the exception of sex. In all 3 groups, rituximab was used most often as the second line of biological treatment but, in group 1, there was a high percentage of patients who received it as first-line therapy. As the effectiveness is usually greater with a first line of treatment and in men, these differences should be taken into account when interpreting the results. There were no differences between first courses and repeated courses in terms of concomitant treatment, although 6% of the patients discontinued corticosteroid therapy.
The results of our study show that when the 3 treatment groups were compared using the IRR, repeated treatments with full doses, as in group 1, were associated with a lower rate of retreatment as opposed to groups 2 and 3, in which the repeat courses consisted of reduced doses. This finding could be due to the fact that treatment with full-dose rituximab achieved longer periods of remission or low disease activity. However, we should not overlook the fact that, in group 1, there was a higher proportion of men and of patients receiving rituximab as first-line biological treatment, although, as we have seen, this did not result in a longer retention time. In fact, the longest rituximab treatment retention time was recorded in group 2, in which the initial course consisted of the full dose and subsequent courses of the reduced dose. These findings may indicate that retreatment courses with the lower dose of rituximab are as effective as courses with the full dose—provided that the only purpose of treatment is to fulfill the objectives of achieving and/or maintaining remission or low disease activity; in addition, a lower rate of adverse effects can be expected.
Group 3 received the highest number of courses, corrected for follow-up, and had the worse rituximab treatment retention. Thus, the administration of reduced doses of 500mg from the beginning may be less effective than starting with a full dose. We can assume that this discrepancy between group 3 and group 2 is due only to the initial dose; therefore, we can hypothesize that, to achieve satisfactory effectiveness, the first course should always consist of 1000mg. The latter point could be the subject of future studies.
We should not lose sight of the fact that the greater the number of courses, the greater the risk of infusion reactions,22 as well as other adverse reactions related to immunosuppression. This was not the case in our study; despite receiving the least number of courses after adjustment for time, it was in group 1 that the highest numbers of both overall and severe adverse effects were recorded. Despite the latter observation, these data suggest that the number of adverse effects does not depend so much on the number of courses, but on the dose utilized in each. In our study, there were fewer overall adverse effects in comparison with other studies.23 This could be due to differences in methodology and the number of patients.
Factors that clearly influence the cost of rituximab are the size of the doses and number of courses required to achieve and maintain complete remission or a low level of disease activity. Despite the differences in the doses and numbers of courses administered in our 3 groups, there were no great differences in the cost. Thus, the impact of this factor should not be considered too important when choosing a treatment regimen.
The limitations of this study are due to its design (retrospective cohort), as well as to the differences between the groups in terms of duration of follow-up and the proportions of men to women and of patients who received rituximab as first-line treatment to those who did not.
Taking into account the above-mentioned limitations, we could conclude that, despite the fact that the patients treated from the start with full doses required a lower rate of repeat treatments/patient-year, the administration of full doses in the first course and reduced doses thereafter appears to be the most effective option, and is only slightly more costly. Reducing the dose does not result in great savings with respect to full doses when compared with the savings produced with administration “on demand”, that is, at the discretion of the treating physician. The rate of adverse effects may be higher in patients treated with full rituximab doses in comparison with those treated with reduced doses, although these differences could be biased, in part because of the higher proportion, in group 1, of patients who received biological agents as first-line treatment. The incidence rate of serious infections is similar to that expected with a biological treatment as the first option.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Mena-Vázquez N, Manrique-Arija S, Ureña-Garnica I, Romero-Barco CM, Jiménez-Núñez FG, Coret V, et al. Eficiencia de diferentes dosis de rituximab en la artritis reumatoide. Reumatol Clin. 2016;12:139–145.