Patients with severe forms of psoriatic arthritis (PsA) usually require treatment with biological agents. A greater knowledge of this subgroup of patients and their treatment enables better decision making in real clinical practice.
MethodsLongitudinal, multicentric observational study. We included all patients older than 16 years diagnosed with PsA in treatment with biological therapies from January 1, 2011 to December 31, 2015 treated in 6 Galician hospitals.
ResultsSix hundred and four patients with PsA received biological therapies. Etanercept was the most used biological treatment. The average time of follow-up was 2.5 years and 67.9% were being treated with the first biological treatment. They were mostly patients with the peripheral subtype and met the criteria for clinical remission. Thirty-two percent had positive HLA-B27 and it was associated with axial PA subtypes.
The prevalence of tuberculosis treated previously was 5.9%, and 23% of patients received chemoprophylaxis for latent tuberculosis. Twenty-four patients had undergone a prosthetic replacement. Hip prosthesis was the most frequent. Ninety-nine cases were treated for affective disorders. A diagnosis of fibromyalgia was established in 11 cases mostly women. Of the cases, 6.6% had episodes of serious infections, with respiratory infections being the most frequent. Sixteen tumours were detected (2.9%). Prostate cancer and gynaecological tumours were the most frequent. As with infections, the greater the age the greater the risk of presenting a tumour.
ConclusionsWe describe the epidemiological and safety characteristics in real life of a Galician multicentre cohort of patients with psoriatic arthritis under biological treatment
Los pacientes con formas graves de artritis psoriásica (APs) habitualmente requieren tratamiento con agentes biológicos. Un mayor conocimiento de este subgrupo de pacientes permite una mejor toma de decisiones en la práctica clínica real.
MétodosEstudio observacional retrospectivo, multicéntrico. Se incluyeron todos los pacientes mayores de 16 años diagnosticados de APs en tratamiento con terapias biológicas desde el 1 de enero de 2011 hasta el 31 de diciembre del 2015.
ResultadosSeiscientos cuatro pacientes con APs recibieron terapias biológicas. El etanercept fue el tratamiento más utilizado. En su mayoría eran pacientes con el subtipo periférico y cumplían criterios de remisión clínica. Un 32% presentaba HLA-B27 positivo y se asociaba a subtipos de APs axial.
La prevalencia de tuberculosis tratada previa fue del 5,9% y el 23% de los pacientes recibió quimioprofilaxis por tuberculosis latente. Veinticuatro pacientes sufrieron una sustitución protésica. La prótesis de cadera fue la más frecuente. Noventa y nueve casos fueron tratados por trastornos afectivos. El diagnostico de fibromialgia fue establecido en 11 y mayormente en mujeres. El 6,6 % de los casos tuvieron episodios de infecciones graves, siendo las infecciones respiratorias las más frecuentes. Se detectaron 16 tumores (2,9%). El cáncer de próstata y los tumores ginecológicos fueron los más frecuentes. Al igual que ocurría con las infecciones a mayor edad mayor riesgo de presentar tumor.
ConclusionesDescribimos las características epidemiológicas y de seguridad en vida real de una cohorte multicéntrica gallega de pacientes con artritis psoriásica en tratamiento biológico.
Psoriatic arthritis (PsA) is a multifactorial chronic inflammatory disease that negatively impacts patient quality of life. Due to its complexity, after its original description by Moll and Wright in the 1970s, with the definition of 5 types of joint involvement,1 different classifications have been proposed for its detection and early diagnosis. The most widely used classification is the one by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (the GRAPPA group). This is based on simple and highly specific disease criteria, with a points system for classification2; this is the classification known as the CASPAR criteria (CLASsification criteria for Psoriatic ARthritis).
According to the response to treatment and impact on quality of life, we establish 3 PsA sub-groups: mild, moderate and severe.3 In the severe forms patients require treatment using disease-modifying drugs (DMARD) and anti-TNF agents or other biological therapies, and their main everyday activities are usually restricted.
In clinical terms there are 5 major areas where characteristic manifestations of PsA occur: peripheral arthritis, vertebral arthritis, dactylitis, enthesitis and cutaneous-ungual manifestations.4 These manifestations may be present in isolation or coincide in a single patient. There is also a growing perception that PsA goes beyond skin and joint involvement, as it has a series of associated comorbidities.5 Obesity, diabetes, cardiovascular disease, hepatotoxicity, metabolic syndrome, malignity and affective disorders of anxiety and depression also make a substantial contribution to the increased morbimortality of these patients. Moreover, treatment including biological therapies may also be associated with adverse effects such as the appearance of severe infections or tumours.5,6 The adverse effects described the most often with biological therapies are upper respiratory tract infections, headaches, an increase in hepatic enzymes and infections in general. There is no solid evidence that the general risk of malignity in patients with psoriasis treated using biological agents is any higher than it is for those treated with DMARD. Current data indicate that there is no increase in the risk of cancer, except for cutaneous carcinomas and, perhaps, melanoma.5 Special mention must be made of the risk and potential severity of tuberculosis in patients under treatment with biological agents.7 All patients who are candidates for such treatment, once infection with tuberculosis has been ruled out, must be monitored to rule out tuberculosis during therapy, as well as for some time afterwards.7
Treating PsA with biological agents is not a new approach. Nevertheless, these therapeutic agents are still in the “new medication” phase, so that the aim of this study is to describe PsA patients treated with biological agents and their complications in our community. It will also underline their epidemiological and clinical characteristics, how their joints are affected, their association with the B27 antigen and related comorbidities or adverse events.
MethodsThis is an observational, longitudinal and retrospective multicentre study. All of the patients gave their informed consent to take part in it. The data gathered were anonymised using an alphanumerical code to preserve complete confidentiality. This study was approved by the Santiago-Lugo Research Ethics Committee. Registry code: 2015/671.
PatientsAll patients were included who were older than 16 years and diagnosed PsA according to the CASPAR2 criteria and treated with biological therapies from 1 January 2011 until 31 December 2015 in 6 Galician hospitals (HULA, CHUO, CHUP, CHUVI, CHUF y CHUAC). These hospitals have a catchment population of 1,746,509 (approximately 60% of the total population of Galicia over the age of 16 years). The degree to which the disease was considered to be severe and the indication for biological therapy were based on clinical management by the doctor and SER8 recommendations.
VariablesPatient data were obtained from their clinical history, either printed or electronic, in the different hospitals where they were treated. A database was designed, which we denominated the Luis Sueiro Database, which contained the demographic characteristics (age and sex) and clinical characteristics of the patients (peripheral, axial or mixed form), the presence of rheumatoid factor, HLA-B27 and past and current treatments. A history of tuberculosis was also recorded at the start of treatment.
During follow-up the following adverse events were specifically recorded: 1) severe infections in any location ―defined as an infection which required hospital treatment and intravenous antibiotic therapy during at least 24 h.―, 2) neoplasm, 3) prosthesis and 4) treatment in a mental health unit for depression or anxiety.
Statistical analysisEach study variable was subjected to descriptive analysis. For continuous variables the core tendency was measured by the average and standard deviation, or the median and interquartile range. Nominal and ordinal variables were expressed as absolute values and proportions. Confidence intervals were estimated using the corrected (Wilson) Score formula.9 Qualitative variables were compared using the chi-squared test (Fisher’s test when necessary) and quantitative variables were compared using the Student t-test, ANOVA, the Mann-Whitney U test or the Kruskal-Wallis test. Rates of incidence per 100 patients/year were estimated for adverse events. Data were analysed using the SPSS 17.0 statistical package, and the confidence intervals of the estimations were analysed using OpenEpi (available at http://www.openepi.com).
ResultsA total of 2310 individuals (1284 men, 55.6%) fulfilled the CASPAR criteria for the diagnosis of PsA during the study period. A total of 604 patients with PsA received biological therapies, distributed as follows among the hospitals: HULA 111 (18.3%), CHUO 60 (9.9%), CHUVI 117 (19.4%), CHUP 72 (11.9%), CHUF 44 (7.4%) and CHUAC 200 (33.1%). Etanercept was the biological treatment used the most often (42%), followed by adalimumab (36%).
Forty-seven per cent of the patients received a biology agent as monotherapy, regardless of the type of therapy used (P = .600). A combination with DMARD was less frequent in patients with axial forms (14.3%; P = .001) and it was more frequent in women (59.0%; P = .039). When a concomitant DMARD was used, methotrexate (41.8%) and leflunomide (11.2%) were used the most often. There were no significant differences between the hospitals that participated in the study respecting this datum.
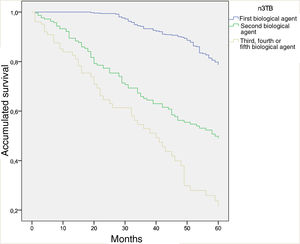
Forty-nine per cent of the patients had been taking biological therapy for from 1 to 5 years, while 30.4% had done so for from 6 to 10 years. 67.9% were receiving their first biological treatment; lack of efficacy (83.5%) was the main reason for switching to a second biological agent. The 5-year survival curve which compares the first biological agent (naive), the second one and the third or more than 3 biological agent shows a clearly significant difference between both curves (P < .001) (Fig. 1). In the opinion of the doctor who treated these patients, the majority of cases (74.1%) are in total remission.
Survival curve at 5 years comparing the first biological agent (naive), the second biological agent and the third or more biological agents (P < .001).
Total treatments: 926.
Total first biological agent: 604. Total second biological agent: 220. Total third or more of 3 biological agents: 102.
The biological therapy was optimised in 33% of cases; golimumab was significantly the one that was least optimised (P = .001). 61.4% of the patients had been receiving biological therapy for less than 5 years; this group was the one with the lowest percentage of optimisation (P < .0001).
Epidemiological characteristicsTable 1 shows the characteristics of the patients included in the study and estimation of the degree to which they are representative of the Galician population. In general, this is a sample with average ages, a similar distribution according to sex and long-term evolution of psoriasis and arthritis, which generally appear around the age of 30–40 years. The majority of arthritis cases were peripheral, and a quarter of them were accompanied by onychopathy and less often by enthesitis, dactylitis or uveitis. HLA-B27 had been determined in 226 patients and it was positive in 73 (32%), and it was associated with PsA subtypes with axial involvement (P = .006).
Estimation of population parameters of the patients with psoriatic arthritis at the start of treatment with biological therapies (Galicia, 2011–2015).
| Characteristic | Parameter | CI 95% |
|---|---|---|
| Age, average in years | 53 | 44.0−62.0 |
| Male sex, n (%) | 313 (55.7) | 51.8−59.9 |
| Years of psoriasis evolution, median | 19 | 5.5−29.0a |
| Age at diagnosis of psoriasis, median | 31 | 21.0−40.0a |
| Years of arthritis evolution, median | 11 | 5.0−18.0a |
| Age at diagnosis of arthritis, median | 39 | 30.5−46.5a |
| Psoriatic onychopathy, n (%) | 128 (22.9) | 19.5−26.5 |
| Enthesitis, n (%) | 84 (15.0) | 12.2−18.1 |
| Dactylitis, n (%) | 34 (6.1) | 4.3−8.3 |
| Uveitis, n (%) | 18 (3.2) | 2.0−5.0 |
| Initial forms, n (%) | ||
| Peripheral | 404 (72.2) | 68.3−75.7 |
| Axial | 47 (8.4) | 6.3−10.9 |
| Mixed | 109 (19.5) | 16.4−22.9 |
| HLA-B27b, n (%) | 73 (32.3) | 26.5−38.7 |
| Previously treated tuberculosis, n (%) | 33 (5.9) | 4.2−8.2 |
| Latent tuberculosis, n (%) | 129 (23.0) | 19.7−26.7 |
All data refer to the moment of starting to take the first biological agent.
Regarding the risk of complications, 5.9% of cases had had previously treated tuberculosis (4.8% of the women and 6.7% of the men). Of all the hospitals, the lowest incidence of this was found in the CHUO, and this was significant (P = .041). According to the recommendations of the SER6 and following screening for tuberculosis, 23% of the patients (21.7% of the women and 23.9% of the men) were treated with chemoprophylaxis. When the incidence of tuberculosis was analysed per hospital, the CHUVI had the lowest incidence (12.9%; P < .001). Three patients receiving biological treatment developed active tuberculosis during the study period.
Disease complicationsThe average duration of follow-up was 2.5 years. The total number of patients/year in follow-up was 1,510.
Table 2 shows the rates of incidence of the complications that were studied. These complications included 24 patients who received a prosthesis (5.2% of the women and 3.5% of the men); the highest number of prostheses corresponded to the CHUO. Hip prostheses were the most frequent (18 cases), followed by those of the knee (12 cases), and there was one elbow prosthesis.
Ninety-six cases (16.4%) were referred to the mental health unit, where they were diagnosed and treated for depression and anxiety. The diagnosis of fibromyalgia was established in 11 cases (3.5%), the majority of which were in women (6.6% in women and 1.1% in men; P < .01).
Thirty-seven episodes of severe infection occurred during biological treatment (6.6%): respiratory infections were the most frequent, followed by those of the soft tissues and complicated urinary tract infection. In regression analysis advanced age was the variable that was associated with infection in a statistically significant way. The age of the patients without severe infection was 52.91 years (CI 95%: 51.83–53.99) and that of those with severe infection was 58.44 years (CI 95%: 54.15–62.74; P = .012; odds ratio1.037; CI 95%: 1.008–1.066).
During the study period 16 tumours (2.9%) were detected. Prostate cancer (6 cases) and gynaecological tumours (3 cases) were the most frequent. Additionally 2 bladder cancers were found, together with 2 cerebral tumours and one tongue cancer. In regression analysis with different co-variables the only factor with a statistically significant association with tumours was age. The age of the patients without neoplasm was 53.08 years (CI 95%: 52.02–54.15) and that of those with neoplasm was 63.40 years (CI 95%: 56.25–70.55; P = .012; odds ratio: 1.073; CI 95%: 1.015–1.134).
DiscussionIn this study we have described the population with PsA who are treated with biological therapies and the main complications of the disease and treatment. These patients were classified as severe PsA for peripheral arthritis, cutaneous disease, axial involvement, enthesitis and dactylitis, according to the criteria proposed by Ritchlin et al.3 The novelty of this study lies in that it is a multicentre study of patients with severe PsA, and that it represents the majority of the Galician population who were treated.
Approximately one quarter of the Galician patients with PsA are currently under treatment with biological therapies. In the International Psoriasis and Arthritis Research Team (IPART) registry, with a total of 7 participating hospitals, a total of 1671 patients with PsA had been included until 2008; 18.4% of these patients were under treatment with biological therapy. The average age of these patients was 46 years (SD 13).10 In the Consortium of Research Rheumatologists of North America (CORRONA) study, of a total of 2426 patients included until 2008 with PsA, 56.2% of these patients received biological therapy.11 The average age of these patients was 54 years (SD 13). In the IPART study the ratio between men and women was 53/47, and in the CORRONA study it was 49/51. The similarity of these data with the findings in our study should be underlined.
The prevalence of enthesitis and dactylitis in our cohort (at 15% and 6%) was lower than rates described previously. The Swedish register of early onset PsA described the incidence of dactylitis to stand at 25% in women and 35% in men.12 Mease et al., in the CORRONA registry, found a prevalence of 27% enthesitis and 15% dactylitis, which are lower than in the Swedish registry.13 The lower incidence of enthesitis and dactylitis in our registry could be explained by the underestimation arising from a retrospective study, as well as infradiagnosis due to the difficulty of diagnosis. Nevertheless, the distribution of the clinical forms is similar to those of other registries, with the multiple joint subtype as the most common.6,14
Sixty-eight per cent of the patients in our registry continued with their first biological treatment. In the biological therapies registry of the Sociedad Española de Reumatología (BIOBADASER) that included 570 patients with PsA, 73% continued with their first anti-TNF treatment at 3 years.15 Subsequent studies have evaluated the survival of anti-TNF treatment in patients with PsA, with similar results. The British registry and the Danish registry of biological therapies describe persistence of treatment with the first anti-TNF agent of 75% and 60%, respectively.16,17 Likewise, a recently published Australian multicentre study of PsA describes a 75.1% survival of biological treatment in monotherapy at 24 months.18 The chief cause of suspension of the first biological treatment was loss of efficacy, as was the case in previous studies.17–19
Sixteen tumours were detected during the follow-up period in 604 patients (2.9%), of which the most frequent were prostate and gynaecological tumours. Several studies evaluated the incidence of neoplasia in 665 patients with PsA in comparison with the historic incidence of cancer in Toronto, and they found 68 neoplasms in 665 patients (10.2%), without any differences respecting the control population.20 The tumours found the most often were of the breast, lungs and prostate. A recent observational study of the British registry of biological therapies evaluated the incidence of cancer in a cohort of patients with severe PsA under biological treatment.21 The results show 34 neoplasms in a population of 709 patients with PsA, in which the majority of cancers were non-melanoma skin cancers, without differences in the overall risk of malignity in comparison with the general population. Subsequent studies have given similar findings, without evidence of an increased risk of neoplasia in patients with PsA respecting the general population or patients with rheumatoid arthritis.22–25 As was the case in our study, increased age is associated with a higher risk of tumours.22,25,26
This study has several limitations. The first is that due to the design of the study data were gathered retrospectively, with the consequent risk of distortions in patient selection and data recording, given that in the majority of cases no activity indexes were recorded. Moreover, as different hospitals were involved and no working protocol had been agreed, there are differences in how patients were evaluated. Additionally, number of patients taking golimumab or certolizumab is reduced by the fact that they were commercialised at a later stage. On the other hand, the lack of a control groups restricts the interpretation of the results.
To conclude, our study describes epidemiological characteristics and data on the efficacy and safety of biological treatments used in a Galician multicentre cohort for patients with PsA. This study is an important source of epidemiological information regarding the actual behaviour of the disease, as well as survival and the long-term safety of treatment using biological therapy.
FinancingThis study was unfinanced and was undertaken using our own resources.
Conflict of interestsThe authors have no conflict of interests to declare.
The authors would like to thank Dr. Alfredo Willisch, rheumatologist of Hospital Universitario, Ourense, for his help in the preparation and revision of this paper. They also wish to thank the Sociedad Gallega de Reumatología for its support for this project.
Please cite this article as: García Porrúa C, Maceiras Pan FJ, Mosquera Martínez JA, Carmona L, Correa Rey B, Fernández Domínguez L, et al. Características epidemiológicas y eventos adversos de los pacientes con artritis psoriásica en tratamiento con terapias biológicas en Galicia. Reumatol Clin. 2021;17:150–154.