Interstitial lung disease (ILD) is a common comorbidity present in patients with systemic sclerosis (SSc). Employment of high-resolution computed tomography (HRCT) is very limited and lung ultrasound (LUS) can be an alternative tool for the early evaluation of ILD.
ObjectiveTo determine the validity of LUS in the early detection of ILD in patients with SSc.
Materials and MethodsSixty-eight patients with SSc ≥18 years without respiratory symptoms were included. A rheumatologist rated the subclinical respiratory condition, another rheumatologist blinded to the clinical assessment performed the LUS. To determine validity HRCT was performed as well.
ResultsPrevalence of LID in SSc patients was 41.2% in contrast to the 4.8% healthy controls (P = .0001). Variables associated with LUS and HRCT findings were anti-centromere antibodies (P = .005) and the Rodnan skin score (P = .004). A positive correlation was present between the findings of HRCT and LUS (P = .001). Sensitivity and specificity were 91.2% and 88.6% respectively. Good reliability in the LUS findings was found between observers (k = .72).
ConclusionBy proving to be a valid, trustworthy and feasible alternative tool, we consider that LUS can be implemented for the early detection of ILD in SSc.
La enfermedad pulmonar intersticial (EPI) es una complicación común de la esclerosis sistémica (ES). El empleo de la tomografía computarizada de alta resolución (TACAR) se ve muy limitado y el ultrasonido pulmonar (USP) puede ser un instrumento alternativo para la evaluación de la EPI.
ObjetivoDeterminar la validez del US pulmonar en la detección temprana de la EPI en pacientes con ES.
MétodosSe incluyeron 68 pacientes con ES ≥ 18 años sin síntomas respiratorios. Un reumatólogo valoró el estado respiratorio subclínico, otro reumatólogo, cegado a la evaluación clínica realizó el USP. Para determinar la validez concurrente se realizó una TACAR.
ResultadosUn 41,2% de pacientes mostraron EPI por USP a diferencia de los controles sanos (4,8%) (p = 0,0001). Las variables asociadas con los hallazgos de EPI al USP fueron anticuerpos anti-centrómero (p = 0,005) y la puntuación de piel RSS (p = 0,004). Se encontró una correlación positiva entre los hallazgos de EPI por USP y TACAR (p = 0,001). La sensibilidad fue del 91.2% y la especificidad de 88,6%. Una buena confiabilidad entre observadores de los hallazgos por USP fue observada (k = 0,72).
ConclusionesAl ser una herramienta alternativa válida, confiable y factible, consideramos que el USP puede ser implementado para la detección temprana de EPI en ES.
Interstitial Lung Disease (ILD) is a clinical entity which affects more than half of patients with systemic sclerosis (SS).1,2 It usually presents before the fourth year of SS, and it is often subclinical or asymptomatic.3,4 Although ILD varies considerably in terms of its severity, it is one of the main causes of death in patients with SS.5,6 It is therefore important to raise awareness of this potential complication, given its considerable impact on the prognosis, quality of life and response to treatment of patients with SS.
Different techniques are currently used to detect ILD in patients with SS; as well as clinical evaluation, tests exist to evaluate respiratory function (RFT) and imaging methods are also used. Although these are the most widely used methods for the detection of ILD, it has recently been proven that respiratory symptoms may be absent in the initial stages of the disease, when ILD has already started to develop. Together with this, complementary tests such as RFT may also suffer from low sensitivity in the detection of early stage ILD, due to the fact that these patients often have no alterations or hardly display any restrictive changes or non/specific obstructions that do not express the magnitude of the disease.7 It is in this context that imaging techniques may play a key role in detecting ILD.
Thoracic radiography is widely used as the first imaging technique for the evaluation of ILD. Nevertheless, it has very low sensitivity in the first stages of the disease, and this widely restricts its use, especially for the evaluation of early changes in ILD.
High resolution computed tomography (HRCT) is a highly sensitive imaging technique that has been proven to be useful in the diagnosis and prognosis of ILD,8,9 and it has the potential to detect pulmonary changes at very early stages.8 In spite of this, routine use of this technique is widely limited by its high costs, its unavailability in health centres and because of the considerable amount of radiation which it emits in each examination.
It has recently been suggested that ultrasound scan (US) of the lung may play an important role in the evaluation of ILD in patients with SS.10–14 All of the works in question took place in patients with SS who already had established ILD, and the technique achieved highly interesting results. For example, a suitable level of correlation was obtained between its findings and those of HRCT, with good reproducibility between operators and above all optimum feasibility.10–14
In spite of the growing evidence for the use of lung US for ILD in patients with SS, to date no accurate data has been available on its role in the detection of early-stage ILD (including asymptomatic or preclinical phases), and above all on whether this instrument could play a predictive role in the evolution of ILD. Given this uncertainty, our team of researchers decided to investigate the validity of pulmonary US in the early detection of ILD.
Material and methodsPatientsConsecutive patients were included in the study who visited the Instituto Nacional de Rehabilitación after being referred by internal and external rheumatologists with a diagnosis of SS based on international criteria.15 The inclusion criteria were: age > 18 years, non-smokers with a diagnosis of SS without respiratory symptoms (dyspnoea and cough). To prevent any superimposition of pulmonary findings patients were excluded if they had a previous diagnosis of ILD, a history of lung disease other than ILD —such as chronic obstructive pulmonary disease or pulmonary oedema due to heart failure or minimum signs of pulmonary hypertension — and if they had ever been subjected to lung surgery. Exclusion due to other causes of ILD was chiefly based on clinical manifestations. Those patients with a minimum suspicion of heart failure or pulmonary hypertension were subjected to an echocardiogram to exclude heart involvement and determine their pulmonary arterial pressure.
Study designIn the following controlled clinical trial all of the patients and healthy control were subjected to complete clinical evaluation by an expert rheumatologist to confirm the absence of respiratory symptoms. Special attention was paid to the detection of slight crepitation or “Velcro sounds” in the bases of the lungs during auscultation. The Borg dyspnoea scale was also applied. The Rodnan skin score and thoracic radiography RFT were only applied to patients with SS on the same day, as was the case for pulmonary US, which was performed by a rheumatologist who is an expert in this technique, and who was blind respecting the clinical evaluation and laboratory test results.
To determine concurrent validity, HRCT was applied to patients with SS within the 7 days after pulmonary US. The operator in this case was also blind respecting clinical and laboratory findings as well as the results of the US. Finally, serological samples were taken from all of the patients to determine anti-centromere and anti-Scl70 antibodies. To determine intraobserver reproducibility 2 rheumatologists with different degrees of experience in US and blind to the clinical analyses evaluated half of the patients (one expert with more than 12 years’ experience in US and one operator with one year of experience in US, who had previously received one month of training in pulmonary US). A control group of healthy subjects paired for age and sex were included, after being recruited from among the medical personnel of the institution and patient family members who visited the US area.
This study was undertaken according to the Helsinki Declaration and local regulations. The local Ethics Committee approved the study as INR/CI/42/15 and the informed consent of all of the patients was obtained.
Evaluation of pulmonary ultrasound scanThe pulmonary US examination was performed in the US area of the musculoskeletal and rheumatic diseases division (INR-LGII) using MyLab Seven (SSAOTE SpA) apparatus equipped with a 5−13 MHz multifrequency lineal transducer. The patients were in supine or almost supine position (with their arms raised and their hands behind their neck) for the anterior and lateral examinations, and in sitting position (with their arms down the sides of their trunk) for the posterior examination. The US parameters for the lungs were as follows: frequency = 10−13 MHz; pulse repetition frequency (RFT) = 1 and wall filter = 4. The examination protocol was based on previously published work.14
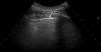
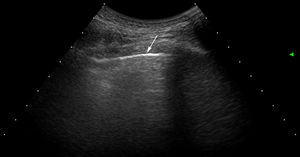
Ultrasound scan interpretationThe basic finding to be evaluated by US is the B line, which is defined as a hyperechoic narrow base reverberation artefact, which extends like a laser beam until the edge of the screen. These B lines are generally not present in the lungs of healthy subjects16,17 (Fig. 1). In each intercostal space (IS) the number of B lines was recorded, and subsequently the total number of all of the B lines found by pulmonary US was registered and classified using the semi-quantitative scale proposed beforehand (0 = normal, ≤ 5 B lines; 1 = mild, ≥ 6 and ≤ 15 B lines; 2 = moderate, ≤ 16 and ≥ 30 B lines; 3 = severe, ≥ 30 B lines). The above operation was performed for the purpose of correlation with HRCT findings.
Evaluation of high resolution computed axial tomographyThe HRCT examination was performed using a standard protocol and a CT Light E Light Speed CT power scanner with a rotation tube with an exploration time of 0.65 s. Scans were obtained with complete aspiration from the lung vertex to its base, with the patients in supine position, at 120 kV and 300 mA, with a slice thickness of 1.25 mm and a spacing of 7 mm. In cases that showed increased opacity in the posterobasal segments a limited number of slices were also acquired through the lower areas of the lung, with the patient in prone decubitus, to ensure that the opacity was not due to gravity-dependent perfusion. HRCT evaluation did not include the use of contrast media agents.
Pulmonary involvement was evaluated by means of lung segments according to the Warrick score18. To precisely correlate the US findings with those of HRCT the following semi-quantitative score was used: (0 = normal [0 points]; 1 = mild [< 8 points]; 2 = moderate [from 8 to 15 points] and 3 = marked [> 15 points]).
Statistical analysisThe Stata v13.0 (StataCorp., TX, U.S.A. statistical package was used for statistical analysis. The descriptive results are expressed as an average and SD and median. Categorical data were expressed as proportions. In multivariate analysis (to determine the associations between variables and the US findings) an unconditional multiple regression logistic model was used to obtain the odds ratios. Spearman’s coefficient was used to correlate US and HRCT. Precision (including sensitivity and specificity) was measured by the area under the ROC curve. Interobserver reliability was calculated by a weighted kappa statistic.19 A P value of .05 was considered statistically significant.
ResultsA total of 77 patients with a diagnosis of SS were referred to be included in the study. During the recruitment phase no patient gave any reason to suspect pulmonary hypertension. Nine patients were excluded, as they had at least of the exclusion criteria. The study was finally undertaken with 68 patients with SS and 68 healthy controls. A total of 952 IS in 68 patients with SS were examined using pulmonary US. The demographic and clinical characteristics of the study population are shown in Table 1. Twenty eight of the 68 patients (41.2%) showed signs of ILD in US, in contrast with the healthy controls (4.8%) (P = .0001). Fig. 2 shows the different signs of ILD detected by US, including comparative US and HRCT images.
Demographic data of the population studied.
| Variable | Average ± SD o (%) |
|---|---|
| Sex | |
| Men | 7 (10.30) |
| Women | 61 (89.70) |
| Age (years) | 51.2 ± 10.2 |
| Disease duration (years) | 4.09 ± 2.3 |
| Type of SS | |
| Limited | 29 (42.6) |
| Diffuse | 39 (57.4) |
| Treatments | |
| None | 26 (38.23) |
| Methotrexate | 18 (26.47) |
| Mycophenolate mofetil | 20 (29.41) |
| Sildenafil | 0 (.00) |
| Azathioprine | 1 (1.47) |
| Bosentan | 1 (1.47) |
| Cyclosporine | 1 (1.47) |
| Cyclophosphamide | 1 (1.47) |
| Raynaud’s phenomenon | |
| Positive | 59 (86.7) |
| Negative | 9 (13.3) |
| Rodnan skin score | 10.9 ± 7.9 |
| Pulmonary auscultation | |
| Positive | 0 |
| Negative | 68 (100) |
| Borg dyspnoea scale | |
| 0 | 66 (97.05) |
| 0.5 | 2 (2.95) |
| Anti-topoisomerase (anti-Scl-70) | |
| Positive | 63 (92.64) |
| Negative | 5 (7.36) |
| Anti-centromere | |
| Positive | 38 (55.88) |
| Negative | 30 (44.22) |
| Thoracic radiography | |
| Positive | 2 (2.95) |
| Negative | 66 (97.05) |
| Pulmonary US (semi-quantitative scale) | |
| 0 | 28 (41.17) |
| 1 | 26 (38.23) |
| 2 | 14 (20.6) |
| 3 | 0 (0) |
| HRCT (semi-quantitative score) | |
| 0 | 27 (39.70) |
| 1 | 30 (44.11) |
| 2 | 9 (13.23) |
| 3 | 2 (2.94) |
| FEV1 | 93 ± 24.5 |
| FVC | 96.9 ± 26.2 |
FEV: Forced Exhalation Volume; FEV 1: Forced exhalation Volume in 1 s; HRCT: High Resolution Axial Computed Tomography.
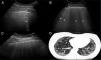
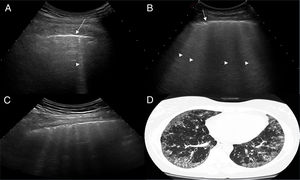
US images showing different signs of ILD based on the number of B lines.
A. There is only one B line (arrowhead). B. 4 B lines are visible (arrowheads). Pleural irregularity is present in both cases (arrows). C. US image showing an intercostal space with 6 B lines and net pleural irregularity in comparison with the HRCT image of ILD (D).
The Rodnan skin score and anticentromere antibodies (P = .004 and P = .005, respectively) were the clinical and analytical variables which showed an association with ILD detected by US. No association was found with sex, age, disease duration, thoracic radiography or RFT (Table 2).
Association between US signs-ILD and variables.
| Variable | OR (CI 95%) | P |
|---|---|---|
| Sex | 2.46 (0.64−9.41) | .178 |
| Age (years) | 1.03 (0.99−1.07) | .107 |
| Anti-topoisomerase (anti-Scl-70) | 2.34 (0.63−8.74) | .205 |
| Anti-centromere | 2.80 (1.37−5.72) | .005 |
| Disease duration (years) | 1.14 (0.98−1.34) | .087 |
| Raynaud’s phenomenon | 0.70 (0.24−1.99) | .501 |
| Rodnan skin score | 1.07 (1.02−1.13) | .004 |
| Borg dyspnoea scale | 77.68 (3.86−156.08) | .086 |
| Thoracic radiography | 1.37 (0.12−15.57) | .796 |
| RFT | 0.98 (0.96−1.01) | .144 |
CI: confidence interval; RFT: Respiratory Function Tests; OR: odds ratio.
A positive correlation was found between the evaluation of US B lines and evaluation using HRCT with the Warrick score (Spearman’s correlation coefficient rho = 0.8022; P = .0001).
The sensitivity and specificity of US in the detection of ILD calculated in comparison with HRCT were 91.2% and 88.6%, respectively, while they scored 2.5% and 98.1%, 8.7% and 98.3%, 27.5% and 77.3% for thoracic radiography, RFT and pulmonary auscultation, respectively. Table 3 shows more details on the sensitivity, specificity, predictive value and the area under the ROC curve.
Sensitivity, specificity, predictive values and area under the ROC curve.
| Sensitivity | Specificity | PPV | NPV | AUC | |
|---|---|---|---|---|---|
| HRCT | |||||
| Thoracic radiography | 2.5 | 98.1 | 66.6 | 40 | .503 |
| Pulmonary auscultation | 8.7 | 98.1 | 87.5 | 41.6 | .503 |
| RFT | 27.5 | 77.3 | 64.7 | 41.4 | .524 |
| Pulmonary US | 91.2 | 88.6 | 92.4 | 87 | .899 |
AUC: Area Under the ROC Curve; NPV: Negative Predictive Value; PPV: Positive Predictive Value; RFT: Respiratory Function Test.
The overall kappa confidence interval values between observers respecting the semi-quantitative US score showed a good agreement between both researchers (k = 0.72). The average time used to perform the evaluation using pulmonary US was 8.6 min (±SD 1.4; range from 6−12 min).
DiscussionDetecting ILD at an early stage is one of the main challenges in improving the quality of life of patients with SS.20–22 HRCT is currently the standard imaging technique for the evaluation of ILD, including diagnosis and prognosis, as it is able to detect early changes in the lungs as well as subclinical lung involvement.20–22 Interesting evidence has emerged in recent years for the potential of pulmonary US to evaluate ILD in patients with SS. Several studies have supplied important information on the correlation between US and HRCT findings in patients who have SS with established ILD.14,23–25 In addition to the above considerations, encouraging data have been published on the excellent performance of pulmonary US in terms of its feasibility and reliability in the evaluation of ILD.13,25–28 All of these factors have made it possible to open an interesting window for research, centring on the use of B lines as new biomarkers for changes caused by ILD in patients with SS.
Although studies continue to be published, to date none of them has focussed on evaluating the value of pulmonary US in the early diagnosis of ILD, and as far as we are aware, this is the first study to centre on this subject.
By using pulmonary US our results show there is a high incidence (41.2%) of subclinical ILD in patients with SS. These data are highly interesting, given that the evaluated patients were asymptomatic in respiratory terms. Additionally, the Borg dyspnoea scale and RFT in these patients showed no initial restrictive and/or obstructive respiratory anomalies (Table 1). This may support the hypothesis that ILD may remain silent for several years prior to the appearance of respiratory symptoms, as has been shown beforehand by some studies with HRCT.20,22 These data lead to reflection on the usefulness of these tools, as they are widely used in screening for ILD in cases of SS, at very early stages.
Moreover, the sensitivity and specificity of pulmonary US to evaluate ILD were similar to those of HRCT, while thoracic radiography, pulmonary auscultation and RFT were found to be deficient in sensitivity and specificity. As expected, a positive correlation was found between the evaluation of pulmonary US B lines and evaluation using the Warrick score. The results in terms of interobserver reliability were also highly significant, as was the feasibility of the technique, given that complete pulmonary examination (pulmonary US) took an average of 8.6 ± SD 1.4 min, which is a relevant datum for the inclusion of pulmonary US in everyday clinical routine.
Additional conclusions may be drawn from more detailed analysis of our results: firstly, thoracic radiography was confirmed to be an imaging technique with zero sensitivity for the evaluation of ILD, especially in its early stages. Secondly, clinical auscultation and RFT may fail to detect early stage ILD. Thirdly, pulmonary US offers many advantages for pulmonary examination; it is a widely/available frontline technique that is low cost and easily accepted by patients. Fourthly, portable apparatus and even apparatus without power Doppler may be sufficient for complete and detailed evaluation of the lungs, as has been shown.28
We are aware that our study has certain limitations. Firstly there is the low number of patients it covers, making precise evaluation of sensitivity and specificity impossible in a way that would support the data presented more rigorously. Secondly, no other functional tests were applied, such as the 6 min walking test or pulmonary diffusion capacity. Thirdly, another limitation was the fact that intraobserver analysis was not performed, chiefly due to factors involving logistics and time. Fourthly, no tests using HRCT, RFT or thoracic radiography were applied to the healthy population, which would have make it possible to measure the sensitivity and specificity of pulmonary US more precisely. On the other hand, another limitation was the absence of analysis to determine whether there were any differences between the treated and untreated patients. Lastly, only the B lines were considered to be an elemental ILD lesion in pulmonary US. Pleural irregularity has recently been proposed to be new lesion in ILD that may be measured using pulmonary US. However, there is still no consolidated knowledge regarding its clinical involvement.
ConclusionsPulmonary US was shown to be a valid, reliable and feasible tool for the early detection of ILD in cases of SS, even in preclinical stages. Although much work remains to be done, the preliminary results of this study may represent a milestone in the path towards the implementation in the near future of pulmonary US as an innocuous screening tool for the very early diagnosis of ILD in cases of SS.
FinancingThis work was supported by a grant from the Research Unit of the Colegio Mexicano de Reumatología (CMR 06/18).
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Reyes-Long S, Gutierrez M, Clavijo-Cornejo D, Alfaro-Rodríguez A, González-Sámano K, Cortes-Altamirano JL, et al. Intersticiopatía pulmonar subclínica en pacientes con esclerosis sistémica. Estudio piloto sobre el papel del ultrasonido. Reumatol Clin. 2021;17:144–149.