Despite the evidence, there are doubts about the positioning of apremilast in the psoriatic arthritis (PsA) treatment algorithm. The objective of this project was to collect the scientific evidence and the experience of a group of rheumatologists who are experts in the management of PsA with apremilast in clinical practice in Spain.
Material and methodsA scientific committee made up of 6 experts proposed 5 clinical scenarios where the evidence on the use of apremilast in PsA was controversial: (i) Efficacy in peripheral PsA; (ii) Efficacy in enthesitis and dactylitis; (iii) Efficacy in PsA with skin involvement; (iv) Comorbidities; and (v) Apremilast safety. After this, a panel of 17 rheumatologists with expertise in PsA management discussed these scenarios and generated a questionnaire with 50 questions and 156 items following the Delphi methodology. This questionnaire was anonymously answered by the panel.
ResultsAfter 2 voting rounds, the panel of experts reached consensus in 93 of the 156 items raised (59.6%) (67 in agreement and 26 in disagreement). The degree of consensus was 53.3% in the area of “Efficacy in peripheral PsA”; 60.0% in “Efficacy in enthesitis and dactylitis”; 50.0% in “Efficacy in PsA with skin involvement”; 57.1% in “Management of comorbidities in patients with PsA”; and 67.3% in “Implications of safety in the use of apremilast”.
ConclusionsThe structured opinion of the experts complements the available evidence and contributes to the establishment of consensual guidelines for the use of apremilast in PsA.
A pesar de la evidencia, existen dudas sobre el posicionamiento de apremilast en el algoritmo de tratamiento de la artritis psoriásica (APs). El objetivo del presente proyecto fue recoger la evidencia científica y la experiencia de un grupo de reumatólogos expertos en el manejo de la APs sobre el uso de apremilast en la práctica clínica en España.
Material y métodosUn comité científico formado por 6 expertos propuso 5 escenarios clínicos donde la evidencia sobre el uso de apremilast en APs era controvertida: (i) Eficacia en APs periférica; (ii) Eficacia en entesitis y dactilitis; (iii) Eficacia en APs con afectación cutánea; (iv) Comorbilidades; y (v) Seguridad de apremilast. Tras esto, un panel de 17 reumatólogos expertos en el tratamiento de la APs discutió estos escenarios y generó un cuestionario con 50 preguntas y 156 ítems según metodología Delphi, el cual fue respondido de forma anónima por los panelistas.
ResultadosTras 2 rondas de votación, el panel de expertos alcanzó el consenso en 93 de los 156 ítems planteados (59,6%) (67 en el acuerdo y 26 en el desacuerdo). El grado de consenso fue del 53,3% en el área de “Eficacia en APs periférica”; del 60,0% en “Eficacia en entesitis y dactilitis”; del 50,0% en “Eficacia en APs con afectación cutánea”; 57,1% en “Manejo de las comorbilidades en pacientes con APs”; y del 67,3% en “Implicaciones de la seguridad en el uso de apremilast”.
ConclusionesLa opinión estructurada de los expertos complementa la evidencia disponible y contribuye al establecimiento de pautas consensuadas para el uso de apremilast en APs.
Psoriatic arthritis (PsA) is a chronic inflammatory disease associated with a significant decline in function and quality of life1. Patients with PsA are at increased risk of obesity, insulin resistance, type 2 diabetes mellitus, hypertension, hyperlipidaemia, and cardiovascular disease2. Patterns of involvement in PsA have several features, including the presence of dactylitis and enthesitis1.
A diagnosis of PsA is an indication for treatment with systemic therapies3. Current therapeutic options for PsA and its complications include4,5 non-steroidal anti-inflammatory drugs, corticosteroids, conventional disease-modifying antirheumatic drugs (DMARDs) (cDMARDs), and biologic drugs6. New drugs with new, more specific mechanisms of action against PsA have been developed in recent years, including synthetic disease-modifying antirheumatic drugs with a specific target, such as apremilast7. Apremilast is an oral phosphodiesterase-4 inhibitor approved to treat PsA, alone or in combination with other DMARDs, in adult patients who have had an inadequate response or intolerance to previous treatment with a DMARD, and to treat moderate to severe chronic plaque psoriasis8. In contrast to non-specific immunosuppressive drugs, apremilast has an immunomodulatory action9, reducing the risk of severe opportunistic infections and reactivation of tuberculosis10.
Although the evidence from clinical studies is strong and consistent, there remain doubts about the positioning of apremilast within the therapeutic algorithm for PsA, as these studies are relatively recent and no direct comparative clinical trials are available4,11. Therefore, information from routine clinical practice is very important to draw strategic conclusions12,13. The aim of this project was to gather scientific evidence and the experience of a group of rheumatologists with expertise in the management of PsA on the use of apremilast in clinical practice in Spain using the Delphi methodology, generating a series of recommendations on the management of PsA with an eminently practical approach aimed at rheumatologists.
Materials and methodsThe study was conducted in 4 phases using the Delphi technique14 modified by Rand/UCLA recommendations15: 1) identification of clinical scenarios of interest by the Scientific Committee overseeing the management of the project; 2) development of the questionnaire by a panel of rheumatology experts; 3) e-mail survey in 2 rounds; and 4) collection and final analysis of the results.
- 1)
The Scientific Committee comprised a national coordinator and 5 regional coordinators11,16–19. Each regional member proposed several clinical scenarios where, due to the lack of consensus, it would be interesting to make therapeutic recommendations on the use of apremilast in PsA. After analysing and discussing the proposals, the following scenarios were chosen: 1) efficacy in peripheral PsA; 2) efficacy in enthesitis and dactylitis; 3) efficacy in PsA with skin involvement; 4) management of comorbidities in patients with PsA; and 5) safety implications in the use of apremilast.
- 2)
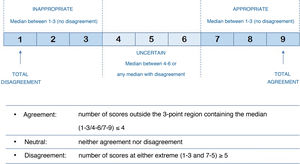
The expert panel for this study was made up of a total of 17 rheumatologists with expertise in the treatment of PsA from different regions of Spain, including the 5 regional coordinators (Appendix A). This panel of experts attended a round table where different cases of apremilast use in routine clinical practice in the 5 proposed scenarios were discussed. This discussion, together with the previous literature review, was the basis for the questionnaire that had 50 questions and a total of 156 items to be assessed. All questions, in the form of statements (affirmative or negative), were designed to be answered on a Likert scale, where 1 meant strongly disagree with the statement and 9 meant strongly agree with the statement (Fig. 1).
- 3)
This questionnaire was sent to the expert panel in electronic format and answered anonymously. After analysing the results of the first round of voting, the items that had not reached consensus in the first round were submitted again to the expert panel for voting. The questionnaire of the second round included the medians of the scores of the first round for each question.
- 4)
Following the Rand/UCLA methodology15, each item’s level of appropriateness was ranked according to the median and the mean of the absolute deviation from the median. Also, following the definitions of the BIOMED Concerted Action on Appropriateness for different panel sizes, the level of agreement for each item was classified as “agree”, “neutral” or “disagree” (Fig. 1). Agreement on an item was determined when at least 77% of the panellists scored within the predefined 3-point interval (Likert score intervals: 1–3, 4–6, 7–9) in which the median score for that item fell. Disagreement on an item was determined when the scores of one third or more of the panellists were in the Likert interval 1–3, and of another third or more in the interval 7−9. Items where there was no agreement or disagreement were considered to have an “uncertain” level of consensus.
The study was conducted over 3 months, from 30 November 2017 to 14 February 2018.
ResultsThe 17 experts on the panel reached consensus in the first round on 58 of the 156 items (37.2%) (46 appropriate and 12 inappropriate). Of the remaining 98 items, consensus was reached on 35 more (21 appropriate and 14 inappropriate) in the second round.
Efficacy of apremilast in peripheral psoriatic arthritisOf the 45 items included in this section of the questionnaire, the experts reached consensus on 24: appropriate in 21 (46.7%; 17 in the first round and 4 in the second round) and inappropriate in 3 items (6.7%; one in the first round and 2 in the second round) (Table 1).
Efficacy of apremilast in peripheral psoriatic arthritis.
| Question | Items | Median | MAD-M | Appropriateness | Agreement | Result |
|---|---|---|---|---|---|---|
| 1 | Depending on site and/or activity, there should be some form of classification of PsA to establish specific therapeutic targets | 8 | .71 | Appropriate | Agreement | Consensus in 1st round |
| 2 | Depending on the associated comorbidities, there should be some form of classification of PsA to establish specific therapeutic targets | 8 | .35 | Appropriate | Agreement | Consensus in 1st round |
| 3 | It is necessary to develop new drug targets to treat PsA | 8 | .82 | Appropriate | Agreement | Consensus in 1st round |
| 4 | Apremilast is a new treatment paradigm for PsA due to its efficacy and safety profile | 8 | .65 | Appropriate | Agreement | Consensus in 1st round |
| 5 | ACR20 criteria (used in RA) is a good parameter to assess disease activity in PsA | 2 | .88 | Inappropriate | Agreement | Consensus in 1st round |
| 6 | The most appropriate indices to assess response in PsA are: | |||||
| DAPSA | 7 | 1.88 | Appropriate | Neutral | No consensus | |
| CPDAI | 5 | 1.06 | Uncertain | Agreement | No consensus | |
| PsARC | 5 | .76 | Uncertain | Agreement | No consensus | |
| PsAJAI | 5 | .82 | Uncertain | Agreement | No consensus | |
| PASDAS | 6 | 1.12 | Uncertain | Neutral | No consensus | |
| AMDF | 5 | 1.00 | Uncertain | Agreement | No consensus | |
| DAS-28 | 4 | .94 | Uncertain | Agreement | No consensus | |
| ACR20 | 3 | .47 | Inappropriate | Agreement | Consensus in 2nd round | |
| HAQ | 5 | 1.12 | Uncertain | Neutral | No consensus | |
| PsAQol | 5 | 1.18 | Uncertain | Neutral | No consensus | |
| MDA | 7 | .88 | Appropriate | Agreement | Consensus in 2nd round | |
| Tender and/or swollen joint count | 7 | .76 | Appropriate | Neutral | No consensus | |
| 7 | In PsA, more realistic parameters of disease activity need to be defined and validated | 8 | .53 | Appropriate | Agreement | Consensus in 1st round |
| 8 | Patient satisfaction with apremilast treatment is a particularly relevant measure when assessing the therapeutic effect of the drug | 7 | .65 | Appropriate | Agreement | Consensus in 2nd round |
| 9 | The variables that most positively influence the choice of apremilast to treat patients with PsA are: | |||||
| Peripheral involvement | 7 | 1.12 | Appropriate | Neutral | No consensus | |
| Axial involvement | 1 | 1.53 | Inappropriate | Agreement | Consensus in 2nd round | |
| Disease activity | 6 | .71 | Uncertain | Agreement | No consensus | |
| Comorbidities | 8 | .47 | Appropriate | Agreement | Consensus in 1st round | |
| Extra-articular manifestations | 5 | .88 | Uncertain | Agreement | No consensus | |
| The patient’s opinion | 7 | .82 | Appropriate | Agreement | Consensus in 2nd round | |
| 10 | Apremilast is a particularly suitable option to treat patients with moderately active PsA after DMARD failure/contraindication | 8 | .65 | Appropriate | Agreement | Consensus in 1st round |
| 11 | In this patient profile, the variables that most positively influence the initiation of treatment with apremilast are: | |||||
| Moderate inflammation | 7 | 1.12 | Appropriate | Agreement | Consensus in 1st round | |
| Stable clinical picture | 5 | 1.29 | Uncertain | Neutral | No consensus | |
| Presence of nail psoriasis, scalp psoriasis or palmoplantar psoriasis | 7 | .88 | Appropriate | Agreement | Consensus in 2nd round | |
| Presence of comorbidities | 8 | .59 | Appropriate | Agreement | Consensus in 1st round | |
| Polymedicated patients | 8 | .71 | Appropriate | Agreement | Consensus in 1st round | |
| Young patients who are expected to require long-term treatment for PsA | 6 | 1.24 | Uncertain | Neutral | No consensus | |
| 12 | The therapeutic target of treatment with apremilast in patients with PsA is: | |||||
| Pain reduction | 7 | 1.00 | Appropriate | Neutral | No consensus | |
| Improvement in the other signs and symptoms of the disease, including skin and nail involvement | 7 | 1.00 | Appropriate | Agreement | Consensus in 1st round | |
| Optimisation of functional capacity and quality of life | 7 | 1.12 | Appropriate | Agreement | Consensus in 1st round | |
| Inhibition of progression of joint damage | 5 | 1.59 | Uncertain | Neutral | No consensus | |
| Preservation of function, prevention of disability and maintenance of the patient’s quality of life | 7 | 1.06 | Appropriate | Neutral | No consensus | |
| Reducing inflammation in domains of the disease such as joints, tendons, entheses, and skin involvement | 8 | .76 | Appropriate | Agreement | Consensus in 1st round | |
| Achieve remission or at least low disease activity | 8 | 1.18 | Appropriate | Agreement | Consensus in 1st round | |
| 13 | It is appropriate to change treatment with apremilast in the absence of a complete clinical response (primary inefficacy) during the first 16 weeks of treatment | 5 | 2.00 | Uncertain | Disagreement | No consensus |
| 14 | In patients with a slow response in the first 16 weeks of treatment with apremilast, it is appropriate to maintain treatment until week 24 and make a further assessment with the agreement of the patient | 8 | .65 | Appropriate | Agreement | Consensus in 1st round |
| 15 | Apremilast is an appropriate option in patients with mildly disabling disease or moderate disease activity and no radiographic progression factors | 8 | .82 | Appropriate | Agreement | Consensus in 1st round |
| 16 | Apremilast is an appropriate option to treat patients with PsA after conventional synthetic DMARD failure or contraindication, who do not yet require or are naïve to biologic therapy | 8 | .59 | Appropriate | Agreement | Consensus in 1st round |
| 19 | In certain circumstances and/or patients, it is appropriate to administer in combination with DMARDs to improve the treatment efficacy for PsA | 6 | .94 | Uncertain | Neutral | No consensus |
| 20 | From a strictly clinical perspective, apremilast is a suitable option to treat patients with highly active PsA in whom biologic therapies are failing/contraindicated | 6 | 1.59 | Uncertain | Neutral | No consensus |
ACR20: 20% improvement in the criteria of the American College of Rheumatology; AMDF: Arithmetic Mean of the Desirability Function; CPDAI: Composite Psoriatic Disease Activity Index; DAPSA: Disease Activity for Psoriatic Arthritis; DAS-28: Disease Activity Score; DMARDs: Disease-modifying Anti-rheumatic Drugs; HAQ: Health Assessment Questionnaire; MAD-M: Mean absolute deviation from the median; MDA: Minimum Disease Activity criterion; PASDAS: Psoriatic Arthritis Disease Activity Score; PsA: Psoriatic Arthritis; PsAJAI: Psoriatic Arthritis Joint Activity Index; PsAQol: Psoriatic Arthritis Quality of Life; PsARC: Psoriatic Arthritis Response Criteria; RA: Rheumatoid Arthritis.
The experts reached consensus that it would be necessary to establish (new) classifications of PsA, according to site, activity, or the presence of comorbidities, to define more specific therapeutic targets (Item 1 [I-1]). Moreover, the panel of experts agreed that, based on current therapeutic options, it would be necessary to explore and develop new pharmacological targets for the treatment of PsA (I-3) and that, in this sense, apremilast, due to its efficacy and safety profile, is a new treatment paradigm for this condition (I-4). Regarding the tools to measure disease activity, the panel agreed that the ACR20 criterion (20% improvement in the American College of Rheumatology criteria) was not an adequate parameter (I-5). Regarding treatment response rates in PsA, the panel reached consensus that the ACR20 criterion, again, was not an appropriate parameter and that, of those suggested, the only criterion appropriate for this purpose would be that of minimal disease activity (MDA) (I-6). They agreed that patient-reported satisfaction would be a particularly relevant measure when assessing the therapeutic effect of apremilast (I-8).
The experts considered apremilast an appropriate option in patients with mildly disabling disease or with moderate disease activity and no radiographic progression factors (I-15), and in patients in whom DMARDs had failed or were contraindicated, including those not medically deemed eligible due to the efficacy/safety profile of biologic therapy (I-10, I-16). In this regard, the experts considered the use of apremilast to be particularly indicated in the following situations: presence of moderate inflammation, presence of nail, scalp or palmoplantar psoriasis, presence of comorbidities, and in polymedicated patients (I-11). In terms of the therapeutic goals for apremilast in PsA, the experts agreed that remission or at least low disease activity should be achieved (I-12).
However, in terms of the response to apremilast, the panel agreed that it would be appropriate, in patients with a slow response in the first 16 weeks, to maintain treatment until week 24 and then reassess, always in agreement with the patient (I-14).
Efficacy of apremilast in enthesitis and dactylitisThe experts reached consensus on 3 (60.0%; all in the second round) of the 5 items included in this section of the questionnaire (Table 2).
Efficacy of apremilast in enthesitis and dactylitis.
| Question | Items | Median | MAD-M | Appropriateness | Agreement | Result |
|---|---|---|---|---|---|---|
| 9 | The variables that most positively influence the choice of apremilast to treat patients with PsA are: | |||||
| Enthesitis | 7 | 1.06 | Appropriate | Agreement | Consensus in 2nd round | |
| Dactylitis | 7 | 1.06 | Appropriate | Neutral | No consensus | |
| 11 | ||||||
| In patients with moderately active PsA after DMARD failure/contraindication, the variables that most positively influence the initiation of treatment with apremilast are: | 7 | .88 | Appropriate | Neutral | No consensus | |
| Presence of dactylitis | 7 | .59 | Appropriate | Agreement | Consensus in 2nd round | |
| 18 | Apremilast is an appropriate option to treat patients with enthesitis and dactylitis, before using biologic therapies | 7 | .82 | Appropriate | Agreement | Consensus in 2nd round |
DMARDs: disease-modifying anti-rheumatic drugs; MAD-M: mean absolute deviation from the median; PsA: psoriatic arthritis.
The experts agreed that the presence of enthesitis is a variable that would positively influence the choice of apremilast for the treatment of patients with PsA (I-9). They also agreed that in patients with moderate PsA in whom cDMARDs had failed or were contraindicated, the presence of dactylitis would positively influence the choice of apremilast as an appropriate option before using biologic therapies (I-11, I-18).
Efficacy of apremilast in psoriatic arthritis with skin involvementThe experts reached consensus on one (50.0%, first round) of the 2 items included in this section of the questionnaire (Table 3).
Efficacy of apremilast in psoriatic arthritis with skin involvement.
| Question | Items | Median | MAD-M | Appropriateness | Agreement | Result |
|---|---|---|---|---|---|---|
| 11 | In patients with moderately active PsA, after DMARD failure/contraindication, the variables that most positively influence the initiation of treatment with apremilast are: | |||||
| Presence of skin involvement | 7 | 1.06 | Appropriate | Neutral | No consensus | |
| 17 | Apremilast is an appropriate option to treat patients with PsA with nail, scalp, palm, and sole involvement, before using biologic therapies | 8 | .82 | Appropriate | Agreement | Consensus in 1st round |
PsA: Psoriatic Arthritis; DMARDs: Disease-modifying Anti-rheumatic Drugs; MAD-M: Mean absolute deviation from the median.
The experts agreed that apremilast would be an appropriate therapeutic option, before using biologic drugs, to treat PsA patients with psoriasis in difficult sites such as nails, scalp or palms and soles of the feet (I-17).
Management of comorbidities in psoriatic arthritis patientsThe experts reached consensus on 28 items: appropriate in 18 (36.7%; 14 in the first round and 4 in the second round) and inappropriate in 10 (20.4%, 5 in the first round and 5 in the second round) of the 49 items included in this section of the questionnaire (Table 4).
Management of comorbidities in patients with psoriatic arthritis.
| Question | Items | Median | MAD-M | Appropriateness | Agreement | Result |
|---|---|---|---|---|---|---|
| 21 | It is not necessary to screen patients before starting treatment with apremilast | 6 | 1.82 | Uncertain | Neutral | No consensus |
| 22 | Patients should be screened before starting treatment with apremilast | |||||
| Before starting treatment with apremilast, a conventional blood test should be performed. | 9 | 1.06 | Appropriate | Agreement | Consensus in 1st round | |
| Before starting treatment with apremilast, a complete blood test including serology for viral infections (VIH, VHB and VHC) should be performed | 7 | 1.12 | Appropriate | Neutral | No consensus | |
| Before starting treatment with apremilast, a chest X-ray should be performed | 4 | 1.24 | Uncertain | Neutral | No consensus | |
| Before starting treatment with apremilast, a test for latent tuberculosis should be performed | 3 | 1.47 | Inappropriate | Neutral | No consensus | |
| Before starting treatment with apremilast in women of childbearing age, a pregnancy test should be performed | 6 | 1.06 | Uncertain | Neutral | No consensus | |
| It is not necessary to change routine vaccination schedules after starting treatment with apremilast | 7 | 1.00 | Appropriate | Neutral | No consensus | |
| 23 | Apremilast is a suitable option to treat patients with PsA and the presence of cardiometabolic comorbidities, before using biologic therapies | 8 | .71 | Appropriate | Agreement | Consensus in 1st round |
| 24 | Apremilast is a therapeutic option that has advantages over other conventional synthetic treatments in patients with PsA with the following comorbidities: | |||||
| Cardiovascular disease | 7 | .65 | Appropriate | Agreement | Consensus in 1st round | |
| Congestive heart failure | 7 | .76 | Appropriate | Neutral | No consensus | |
| Dyslipidaemia | 7 | .53 | Appropriate | Neutral | No consensus | |
| Type 2 diabetes mellitus | 7 | .53 | Appropriate | Neutral | No consensus | |
| Obesity | 8 | .76 | Appropriate | Agreement | Consensus in 1st round | |
| Metabolic syndrome | 7 | .94 | Appropriate | Agreement | Consensus in 1st round | |
| Hepatic impairment | 7 | 1.00 | Appropriate | Agreement | Consensus in 1st round | |
| Chronic renal failure | 7 | .65 | Appropriate | Neutral | No consensus | |
| Demyelinating diseases | 8 | .71 | Appropriate | Agreement | Consensus in 2nd round | |
| Inflammatory bowel disease | 6 | 1.18 | Uncertain | Neutral | No consensus | |
| Mood disorders | 2 | .88 | Inappropriate | Agreement | Consensus in 2nd round | |
| Multicomorbidities | 8 | .65 | Appropriate | Agreement | Consensus in 1st round | |
| 25 | Apremilast is a therapeutic option that has advantages over other biologic treatments in patients with PsA with the following comorbidities: | |||||
| Cardiovascular disease | 7 | 1.24 | Appropriate | Agreement | Consensus in 1st round | |
| Congestive heart failure | 8 | .82 | Appropriate | Agreement | Consensus in 1st round | |
| Dyslipidaemia | 7 | .29 | Appropriate | Agreement | Consensus in 2nd round | |
| Type 2 diabetes mellitus | 6 | .65 | Uncertain | Agreement | No consensus | |
| Obesity | 8 | .65 | Appropriate | Agreement | Consensus in 2nd round | |
| Metabolic syndrome | 7 | .47 | Appropriate | Agreement | Consensus in 2nd round | |
| Hepatic impairment | 7 | .82 | Appropriate | Neutral | No consensus | |
| Chronic renal failure | 6 | .65 | Uncertain | Agreement | No consensus | |
| Demyelinating diseases | 8 | .53 | Appropriate | Agreement | Consensus in 1st round | |
| Inflammatory bowel disease | 5 | 1.47 | Uncertain | Neutral | No consensus | |
| Mood disorders | 2 | 1.29 | Inappropriate | Agreement | Consensus in 1st round | |
| Multicomorbidities | 8 | .47 | Appropriate | Agreement | Consensus in 1st round | |
| 26 | Before starting treatment with apremilast, special attention should be paid to the diagnosis/treatment of the following co-morbidities: | |||||
| Hypertension | 2 | 1.18 | Inappropriate | Agreement | Consensus in 1st round | |
| Type 2 diabetes mellitus | 2 | .94 | Inappropriate | Agreement | Consensus in 1st round | |
| Dyslipidaemia | 3 | 1.00 | Inappropriate | Agreement | Consensus in 1st round | |
| Demyelinating diseases | 2 | .65 | Inappropriate | Agreement | Consensus in 1st round | |
| Ischaemic heart disease | 3 | .88 | Inappropriate | Neutral | No consensus | |
| Cardiovascular accidents | 3 | .88 | Inappropriate | Agreement | Consensus in 2nd round | |
| Peptic disease | 3 | 1.12 | Inappropriate | Neutral | No consensus | |
| Malignant tumours | 3 | .94 | Inappropriate | Agreement | Consensus in 2nd round | |
| Chronic renal failure | 5 | .71 | Uncertain | Agreement | No consensus | |
| Liver disease | 3 | .94 | Inappropriate | Neutral | No consensus | |
| Infections | 4 | 1.12 | Uncertain | Neutral | No consensus | |
| Oral anticoagulation | 3 | .76 | Inappropriate | Agreement | Consensus in 2nd round | |
| Heart failure | 2 | .94 | Inappropriate | Agreement | Consensus in 2nd round | |
| Inflammatory bowel disease | 4 | 1.00 | Uncertain | Neutral | No consensus | |
| Mood disorders | 8 | 1.41 | Appropriate | Agreement | Consensus in 1st round | |
| 31 | The risk/benefit ratio of drugs with potentially negative effects on common comorbidities in PsA should be considered before starting treatment with apremilast | 8 | 1.41 | Appropriate | Agreement | Consensus in 1st round |
| 32 | Early screening and appropriate periodic monitoring of cardiovascular risk is appropriate in patients with PsA | 8 | 1.00 | Appropriate | Agreement | Consensus in 1st round |
DMARDs: Disease-modifying Anti-rheumatic Drugs; HBV: Hepatitis B virus; HCV: Hepatitis C virus; HIV: Human Immunodeficiency Virus; PsA: Psoriatic Arthritis; MAD-M: Mean absolute deviation from the median.
The experts agreed that, in general, before starting treatment with apremilast in patients with PsA, a conventional blood test should be performed (I-22). They also considered it appropriate to undertake early screening and appropriate periodic monitoring of cardiovascular risks in patients with PsA at least once every 5 years, or after each change of treatment (I-32, I-33). They also agreed to define apremilast as an appropriate therapeutic option, before biologic treatments, in patients with cardiometabolic comorbidities (I-23). It would also be appropriate in patients with hepatic impairment versus other cDMARDs and in the treatment of patients with a history of congestive heart failure and dyslipidaemia versus biologic therapies (I-24, I-25). In terms of the comorbidities in patients with PsA requiring special attention before starting treatment with apremilast, there was agreement (consensus in disagreement) that it would not be necessary with arterial hypertension, type 2 diabetes mellitus, dyslipidaemia, demyelinating diseases, cardiovascular accidents, neoplasms, or heart failure. Special attention should be paid to patients showing symptoms of mood disorders, especially those with depressive symptoms with or without antidepressant treatment (I-26, I-47).
Finally, they agreed that the benefit/risk ratio of drugs with potentially negative effects on the common comorbidities of PsA should be considered before starting treatment with apremilast (I-31).
Safety implications in the use of apremilastThe experts reached consensus on 37 items: appropriate in 24 (43.6%; 14 in the first round and 10 in the second round) and inappropriate in 13 (23.6%; 6 in the first round and 7 in the second round) of the 55 items included in this section of the questionnaire (Table 5).
Safety implications in the use of apremilast.
| Question | Items | Median | MAD-M | Appropriateness | Agreement | Result |
|---|---|---|---|---|---|---|
| 27 | Drug monitoring with apremilast not being necessary is a favourable aspect, compared to biologic therapies, in the choice of drug to treat PsA | 8 | .76 | Appropriate | Agreement | Consensus in 1st round |
| 28 | No monitoring of patients during treatment with apremilast is necessary | 5 | 1.94 | Uncertain | Disagreement | No consensus |
| 29 | Some monitoring of patients during treatment with apremilast is necessary: | |||||
| During treatment with apremilast, a complete blood count should be conducted once or twice a year | 8 | 1.24 | Appropriate | Agreement | Consensus in 1st round | |
| During treatment with apremilast testing for latent tuberculosis should be repeated annually | 2 | 1.35 | Inappropriate | Agreement | Consensus in 1st round | |
| Laboratory or other complementary tests should only be performed depending on the patient’s underlying disease | 7 | 2.24 | Uncertain | Disagreement | No consensus | |
| 30 | During treatment with apremilast, the following co-morbidities should be monitored: | |||||
| Hypertension | 2 | .41 | Inappropriate | Agreement | Consensus in 2nd round | |
| Type 2 diabetes mellitus | 2 | .94 | Inappropriate | Agreement | Consensus in 1st round | |
| Dyslipidaemia | 2 | 1.00 | Inappropriate | Agreement | Consensus in 1st round | |
| Demyelinating diseases | 2 | 1.12 | Inappropriate | Agreement | Consensus in 1st round | |
| Ischaemic heart disease | 2 | .47 | Inappropriate | Agreement | Consensus in 2nd round | |
| Cardiovascular accidents | 3 | .65 | Inappropriate | Agreement | Consensus in 2nd round | |
| Peptic disease | 3 | .65 | Inappropriate | Agreement | Consensus in 2nd round | |
| Malignant tumours | 2 | 1.00 | Inappropriate | Agreement | Consensus in 2nd round | |
| Chronic renal failure | 5 | 1.00 | Uncertain | Neutral | No consensus | |
| Liver disease | 3 | 1.00 | Inappropriate | Neutral | No consensus | |
| Infections | 4 | 1.24 | Uncertain | Neutral | No consensus | |
| Oral anticoagulation | 3 | .88 | Inappropriate | Neutral | No consensus | |
| Heart failure | 3 | .94 | Inappropriate | Agreement | Consensus in 2nd round | |
| Inflammatory bowel disease | 5 | 1.18 | Uncertain | Neutral | No consensus | |
| Mood disorders | 8 | 1.24 | Appropriate | Agreement | Consensus in 1st round | |
| 33 | It is appropriate to perform a cardiovascular risk assessment at least once every 5 years, or after every change of treatment, in all patients with PsA | 8 | 1.06 | Appropriate | Agreement | Consensus in 1st round |
| 34 | The use of apremilast as monotherapy is an advantage over biologics in terms of drug persistence | 7 | .65 | Appropriate | Neutral | No consensus |
| 35 | Apremilast is a particularly appropriate option to treat polymedicated patients due to low drug-drug interactions | 8 | .76 | Appropriate | Agreement | Consensus in 1st round |
| 36 | Apremilast is a particularly appropriate option for patients who are scheduled for surgery | 7 | .94 | Appropriate | Agreement | Consensus in 1st round |
| 37 | Apremilast is a particularly suitable option for women of childbearing age who wish to become pregnant | 5 | 1.65 | Uncertain | Disagreement | No consensus |
| 38 | Apremilast is a particularly suitable option for patients with injection phobia | 9 | .59 | Appropriate | Agreement | Consensus in 1st round |
| 39 | One of the most relevant features of apremilast is its good safety profile | 8 | .41 | Appropriate | Agreement | Consensus in 1st round |
| 40 | Apremilast can be considered a safer therapeutic option than conventional systemic treatments | 8 | .47 | Appropriate | Agreement | Consensus in 1st round |
| 41 | as it is not immunosuppressive, apremilast is a more appropriate option than other biologic treatments in patients with any of the following characteristics: | |||||
| Recurrent or chronic infections, including HIV, HBV, HCV, TB infection | 8 | .82 | Appropriate | Agreement | Consensus in 1st round | |
| Recurrent or chronic infections, including HIV, HBV, HCV, Tuberculosis | 8 | .76 | Appropriate | Agreement | Consensus in 2nd round | |
| A recent history of neoplasms (<5 years) | 8 | .47 | Appropriate | Agreement | Consensus in 1st round | |
| Active neoplasia | 7 | .82 | Appropriate | Agreement | Consensus in 2nd round | |
| Immunosuppressed states | 8 | 1.06 | Appropriate | Agreement | Consensus in 1st round | |
| 42 | Weight loss observed during treatment with apremilast is: | |||||
| Only a relevant effect in thin, underweight patients | 6 | 2.06 | Uncertain | Disagreement | No consensus | |
| A potentially beneficial effect in overweight/obese patients (possible risk factors for metabolic syndrome) | 8 | .47 | Appropriate | Agreement | Consensus in 2nd round | |
| 43 | During treatment with apremilast, all patients’ weight should be regularly monitored | 6 | 1.53 | Uncertain | Neutral | No consensus |
| 44 | During treatment with apremilast, specific periodic weight monitoring should be performed only in thin patients who are underweight | 7 | .59 | Appropriate | Agreement | Consensus in 2nd round |
| 45 | Mood impairment is a limiting factor in the choice of apremilast treatment in clinical practice | 7 | 1.18 | Appropriate | Neutral | No consensus |
| 46 | During treatment with apremilast it is advisable to regularly specifically monitor mood in all patients | 7 | .53 | Appropriate | Neutral | No consensus |
| 47 | During treatment with apremilast it is advisable to periodically specifically monitor mood only in patients with depressive symptoms and/or on treatment with antidepressants | 8 | .82 | Appropriate | Agreement | Consensus in 2nd round |
| 48 | Gastrointestinal effects of apremilast are a limiting factor in the choice of apremilast treatment in clinical practice | 6 | .82 | Uncertain | Agreement | No consensus |
| 49 | The most appropriate therapeutic management of patients with gastrointestinal adverse effects is: | |||||
| Reduce fluid intake | 2 | 1.06 | Inappropriate | Agreement | Consensus in 2nd round | |
| Administer apremilast with meals | 6 | 1.24 | Uncertain | Neutral | No consensus | |
| Avoid caffeinated beverages | 5 | 1.35 | Uncertain | Neutral | No consensus | |
| Administer antidiarrhoeals, antiemetics and/or analgesics in cases where the adverse effect is clinically relevant and conditions the continuation of treatment with apremilast | 7 | 1.29 | Appropriate | Neutral | No consensus | |
| Perform a slower induction phase | 8 | 1.00 | Appropriate | Agreement | Consensus in 1st round | |
| Temporarily discontinue the morning dose in cases where the adverse effect is clinically relevant and conditions continuation of treatment with apremilast, resuming treatment once the adverse effect has subsided | 7 | 1.12 | Appropriate | Agreement | Consensus in 2nd round | |
| Temporarily discontinue the morning dose in cases where the adverse effect is clinically relevant and conditions continuation of treatment with apremilast, resuming treatment once the adverse effect has subsided | 7 | 1.12 | Appropriate | Agreement | Consensus in 2nd round | |
| Administer prophylactic antidiarrhoeals, antiemetics and/or analgesics at the start of treatment to all patients | 2 | 1.12 | Inappropriate | Agreement | Consensus in 1st round | |
| Administer antidiarrhoeals, antiemetics and/or analgesics only in cases where the adverse effect is clinically relevant | 7 | 1.12 | Appropriate | Agreement | Consensus in 2nd round | |
| 50 | The most appropriate therapeutic management of patients presenting with headache is: | |||||
| Perform a slower induction phase | 7 | .71 | Appropriate | Agreement | Consensus in 2nd round | |
| Temporarily discontinue the morning dose in cases where the adverse effect is clinically relevant and conditions the continuation of treatment with apremilast, resuming treatment once the adverse effect has subsided | 7 | .82 | Appropriate | Agreement | Consensus in 2nd round | |
| Temporarily discontinue treatment in cases where the adverse effect is clinically relevant and conditions the continuation of treatment with apremilast, resuming treatment once the adverse effect has subsided | 6 | 1.12 | Uncertain | Neutral | No consensus | |
| Prophylactically administer analgesics at the start of treatment to all patients | 2 | .76 | Inappropriate | Agreement | Consensus in 1st round | |
| Administer analgesics only in cases where the adverse event is clinically relevant | 8 | .88 | Appropriate | Agreement | Consensus in 1st round |
PsA: Psoriatic Arthritis; DMARDs: Disease-modifying Anti-rheumatic drugs; MAD-M: Mean absolute deviation from the median; HBV: Hepatitis B virus; HCV: Hepatitis C virus; HIV: Human Immunodeficiency Virus.
The experts agreed that pharmacological monitoring with apremilast not being necessary is an advantage over biologic therapies (I-27). In terms of patient follow-up, they agreed that it would be appropriate to perform a complete blood test once or twice a year, but inappropriate to repeat tuberculosis screening tests annually (I-29).
The experts agreed that it is a particularly suitable option to treat polymedicated patients, needle phobic patients, and those with surgery scheduled (I-35, I-36, I-38).
The experts agreed that one of the most relevant features of apremilast was its good safety profile and that it could be considered a safer option than biological therapies, especially in patients with recurrent, chronic or active infections (including human immunodeficiency virus infection, hepatitis B virus, hepatitis C virus and tuberculosis), a recent history of malignancy (<5 years), active malignancy or immunosuppressed states (I-41).
The expert panel agreed that the most appropriate management of patients experiencing gastrointestinal adverse effects or headaches with apremilast would include considering it inappropriate to reduce fluid intake (in the case of gastrointestinal adverse effects) and administering all patients antidiarrhoeals, antiemetics and/or analgesics prophylactically at the start of treatment. They considered a slower induction phase appropriate, and temporarily discontinuing treatment in cases where the adverse effect is clinically relevant and affects the continuity of treatment with apremilast, resuming treatment once the adverse effect has subsided, and administering antidiarrhoeals, antiemetics and/or analgesics only in cases where the adverse effect is clinically relevant (I-49, I-50).
DiscussionConsensus recommendations based on expert opinion and developed using validated and rigorous methodologies produce useful guidelines, providing experts with valuable specific information. In this study, the Delphi methodology14 was used to achieve consensus and generate recommendations for rheumatologists in the management of PsA. The expert participation was good, with a 100% response rate in the 2 rounds.
According to the experts, the appropriate patient for treatment with apremilast would have moderately active, mildly disabling PsA, including those with skin involvement in difficult sites and other comorbidities commonly associated with the disease, with an inadequate response or contraindication to cDMARDS and not yet candidates for biologic therapies.
The presence of enthesitis and dactylitis, as well as psoriasis in difficult sites, would be factors that would positively influence the use of apremilast before that of biologic therapies. In the PALACE 1–3 studies18,20,21, which assessed the safety and efficacy of apremilast in adult patients with active PsA despite prior treatment with cDMARDs or biologics, improvements in peripheral activity parameters characteristic of PsA were observed at weeks 16 and 24. Apremilast also demonstrated greater ACR20 responses in patients who had not previously been exposed to biologic therapy21.
Regarding the items relating to the use of apremilast versus cDMARDs, the consensus among experts is in line with the approved indication for the drug, which recommends the use of apremilast in patients who have had an inadequate response or in whom there are contraindications to the cDMARDs8. In the PALACE study, the responses observed in the group treated with apremilast were similar between the patients who were receiving and those not receiving concomitant cDMARDs, including methotrexate. In addition, a greater ACR20 response was observed at week 16 in patients previously treated with cDMARDs or biologics receiving apremilast compared to patients receiving placebo8.
Regarding the use of apremilast versus biologic therapies, there was consensus among experts that apremilast is an appropriate choice in patients naïve to biologic therapies. Biologic therapies have an activity ceiling of around 60%22–26, which means that up to 40% of patients with PsA, at best, do not respond to initial treatment. Furthermore, these drugs have immunosuppressive activity, which can generate problems of immunogenicity or toxicity, depending on the patient22–26. In this regard, and due to their immunomodulatory action, the risk of serious opportunistic infections and reactivation of tuberculosis in patients treated with apremilast is lower than with other immunosuppressive drugs10 and does not require screening for tuberculosis8. Another advantage of apremilast over biologic therapies is that it does not require extensive drug monitoring8, which is consistent with the results of previous studies that concluded that there is no need for prior laboratory analysis or continuous monitoring of laboratory parameters27.
Regarding the management of comorbidities, the experts considered that apremilast would be an appropriate therapeutic option in patients with PsA and other comorbidities commonly associated with the disease. Furthermore, no prior screening or follow-up for most of the comorbidities commonly associated with PsA would be required, which is an advantage over cDMARDs and biologics.
Regarding the items relating to the safety of apremilast, in general, the experts' responses are in line with the results obtained in previous studies18,20,21, which demonstrated the efficacy of apremilast in PsA, with a very favourable safety and tolerability profile, and a very convenient dosage and form of administration27,28. The experts agreed that one of the most relevant features of apremilast was its good safety profile and that it could be considered a safer therapeutic option than conventional systemic treatments. The experts also agreed on the management of gastrointestinal adverse effects and headaches, 2 of the most common treatment-related adverse effects8.
In conclusion, apremilast, due to its efficacy, safety, and tolerability profile, is a new therapeutic option for patients with psoriasis and PsA, especially patients with comorbidities, including infections and/or neoplasms, and covers some hitherto unmet needs10,29,30.
FundingCelgene collaborated in organising working meetings and bibliographic support, elaboration and implementation of the Delphi method undertaken by the independent scientific consultancy.
Amgen funded this manuscript.
Conflict of interestsJCTA has participated in presentations, consultancies and educational programmes at Amgen, Lilly, Novartis, Janssen and Pfizer. RAG has participated in presentations, consultancies, attending conferences, courses and educational programmes at AbbVie, Amgen, Gebro, Janssen, Lilly, MSD, Novartis, Pfizer and UCB. CMM has participated in presentations, consultancies, attending conferences, courses, and educational programmes at Amgen, Janssen, Lilly, Pfizer and UCB. JSS has participated in presentations, consultancies, attending conferences, courses and conducting educational programmes at AbbVie, Janssen, Lilly, Pfizer, Novartis, Celgene, and MSD. EPA is an employee and shareholder of Amgen S.A. JG has received funding in relation to courses and/or conferences, has acted as a speaker and/or has been a consultant on occasion for MSD, Pfizer, AbbVie, Janssen, Cilag, Novartis, Celgene, and Lilly.
We would like to thank all the coordinators and participants in the project. Cristina Ceballos, TFS S. L., provided support in drafting the manuscript.
Raquel Almodóvar González
Emma Beltrán Catalán
Ricardo Blanco Alonso
Antonio Rafael Cáliz
Federico Díaz González
Eva Galíndez Aguirregoicoa
María Teresa González Hernández
Jordi Gratacós Masmitja
Cristina Hidalgo Calleja
José Rafael Ivorra Cortés
Ana María Laiz Alonso
Carlos Montilla Morales
Isabel de la Morena Barrio
Deseada Palma Sánchez
Natalia Palmou Fontana
Lucía Ruiz Gutiérrez
Jesús Sanz
Please cite this article as: Torre Alonso JC, Almodóvar González R, Montilla Morales C, Sanz Sanz J, Díaz González F, Pascual Alfonso E, et al. Recomendaciones de expertos para el uso de apremilast en artritis psoriásica. Reumatol Clín. 2023;19:34–44.