To analyse factors involved in the decision to optimise biologics in juvenile idiopathic arthritis.
MethodsA “discrete-choice” methodology was used. In a nominal group meeting, factors which may influence physicians’ decisions to optimise biological dose were identified, together with decision nodes. 1000Minds® was used to create multiple fictitious clinical scenarios based on the factors identified, and to deploy surveys that were sent to a panel of experts. These experts decided for each item which of two clinical scenarios prompted them to optimise the dose of biologic. A conjoint analysis was carried out, and the partial-value functions and the weights of relative importance calculated.
ResultsIn the nominal group, three decision nodes were identified: (1) time to decide; (2) to maintain/reduce or prolong interval; (3) what drug to reduce. The factors elicited were different for each node and included patient and drug attributes. The presence of macrophage activation syndrome (MAS), systemic involvement, or subclinical inflammation made the decision easier (highest weights). The presence of joints of difficult control and year of debut influenced the decision in some but not all, and in different directions. Immunogenicity, adherence, and concomitant treatments were also aspects taken into account.
ConclusionsThe decision to optimise the dose of biological therapy in children and youngster can be divided into several nodes, and the factors, both patient and therapy-related, leading to the decision can be detailed. These decisions taken by experts may be transported to practice, study designs, and guidelines.
Analizar los factores que intervienen en la decisión de optimizar el biológico en la artritis idiopática juvenil.
MétodosSe utilizó la metodología de «elección discreta». Mediante grupo nominal se identificaron factores potencialmente influyentes en la decisión de optimizar la dosis de biológico y los nodos de decisión. Con 1000Minds® se crearon escenarios clínicos ficticios basados en los factores identificados que se mostraron en encuestas a un panel de expertos. Cada ítem de las encuestas mostraba 2 escenarios clínicos y los expertos elegían el que les llevaría a optimizar el biológico. Se realizó un análisis conjunto, calculándose las funciones de valor parcial y los pesos de importancia relativa.
ResultadosSe identificaron 3 nodos de decisión: 1) dilatar decisión o no; 2) mantener/reducir o prolongar el intervalo; y 3) qué fármaco reducir. Los factores identificados varían por nodo e incluyen atributos del paciente y del fármaco. La presencia del síndrome de activación macrofágica, la afectación sistémica o la inflamación subclínica facilitaron la decisión (pesos más altos). La presencia de articulaciones de difícil control y el año de inicio influyeron en la decisión en algunos casos, pero no en todos, y en diferentes direcciones. La inmunogenicidad, la adherencia y los tratamientos concomitantes también fueron aspectos decisivos.
ConclusionesLa decisión de optimizar la dosis de biológico en artritis idiopática juvenil se divide en varios nodos y se pueden detallar factores, tanto del paciente como del tratamiento, que determinan la decisión. Estas decisiones de experto pueden transportarse a la práctica, la investigación y las recomendaciones.
During the last decades, the treatment and prognosis of patients with juvenile idiopathic arthritis (JIA) have changed dramatically. The introduction of biologic agents coupled by the implementation of agile strategies for monitoring and taking decisions are behind the success.
Biological dose reduction – also called tapering, de-escalation, adjustment, optimisation, or spacing – is a widespread practice among paediatric rheumatologists.1 However, the evidence on the maintenance of remission or long-term effects after tapering biologics in JIA is scarce.2 In addition, this decision, taken in clinical practice, is subject to large variability.1,3
Previous experiences on how to taper biological therapies in adults, such as the one carried out by the Spanish Society of Rheumatology (SER) with the REDOSER consensus, have identified patient but not drug-related attributes leading to appropriate tapering.4 The main concern of tapering is relapse, which is not negligible.5 Some of the factors that may play a role in maintaining remission after dose reduction include length of remission, or the absence of subclinical disease.3,6–8
The problems of the paediatric rheumatic population treated with biologics are different from the adult rheumatic population in terms of dosage, efficacy, or the time remaining on the medication, for instance. How to make a dose adjustment does not raise serious questions of evidence, but rather of pharmacokinetics and presentation, which could be extrapolated from adults. However, two more specific problems do arise. On the one hand the reasons—and fears or reticence—for which a paediatrician would initiate dose reduction, and on the other, whom should be reduced, taking into account prognostic factors.
Discrete-choice experiments, or conjoint analysis, are a methodology employed in health economics to quantify preferences for medicines and services but can also be used to understand patient preferences for health states, willingness to accept therapeutic risks, and decision making, and can also be used to understand clinical decision making.9 These methods have been previously used in Rheumatology, among others, to comprehend patient preferences10 and to understand the decision to escalate care in rheumatoid arthritis,11 and so our team thought it could be an adequate methodology to disentangle the decision to taper down biologics in JIA.
The aim of this initiative was thus to identify factors involved in the decision to taper disease modifying drugs (DMARD), being these biological or not, in children and youngsters with JIA, and to quantify their value in the clinical decision-making.
MethodsThis is a mixed-methods study. The methodological approach was carried out in different and successive phases: (1) creation of an expert panel that underwent nominal group techniques to elaborate the conceptual model of the dose reduction process, and to start defining factors involved in this process; (2) ratification and addition of factors or levels of factors by a broader group; and (3) discrete-choice experiment to calculate the utility and weights that physicians assign to the factors involved in reducing or not the dose of DMARDs (Fig. 1).
Expert's selection and nominal groupA scientific committee was established with a national representative panel of specialists with proven expertise in JIA management. The selection of experts (n=17) was based on their membership of the scientific committee of the Spanish Society of Paediatric Rheumatology (SERPE) and for being heads of the paediatric rheumatology units of university hospitals of recognised prestige and experience. In a face to face meeting with methodologists, nominal group techniques were used to define the process of dose reduction, or interval spacing, in children with JIA in treatment with biologics and their decision nodes. Nominal group techniques imply preparing the concept (in this case, the process of adjusting biologics) before the meeting, having time to think in silence during the meeting, writing down thoughts, and then sharing them out loud. A methodologist expert in this technique made sure that everybody had a chance to present their thoughts and to express their opinions about the others’. The meeting was organised in two parts, one to understand the process of drug adjustment, and the second to detail what things would change the mind of the expert when adjusting. Brainstorm dynamics were used to elicit factors that could determine dose adjustment at each decision node. The methodologist encouraged discussions, item by item, to make sure the experts had experienced a case, or could think of one, in which a specific patient or drug factor changed their mind of adjusting the dose or not. In addition, potential levels or categories for each factor were started to be defined (e.g., if age were a factor, the levels could be “2 years of age or under” and “over 2 years”). This methodology does not require recording or transcription of discourse. The process map and all items were presented in real time during the meeting in whiteboards and in mind maps to be cross-checked with the experts and notes were taken.
Ratification by a broader groupAfter the first meeting, the map, nodes, and factors were explained to a broader group of ≈50 paediatric rheumatologists during a national meeting of the paediatric rheumatology group, at which the previous factors or attributes were ratified, more factors were collected, these were better defined, and levels of the factors further specified.
In both meetings all decisions to include or not to include were taken by unanimity.
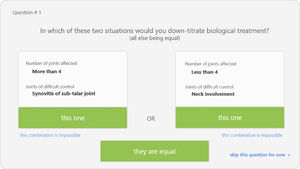
Discrete-choice experiment (DCE)This methodology allows to understand the factors that influence the physician's preferences at the time of reducing or spacing the doses. DCE consists of a survey with several screens presenting two hypothetical clinical scenarios in which the cases are defined each by two alternatives (defined by the factors elicited in previous phases) with varying attribute levels (the categories of factors previously defined). An example of these scenarios is shown in Fig. 2. At each pair of scenarios respondents had to decide in which case, or both or none, they would take the decision. Three DCE surveys were created using the 1000Minds® software. Each of the surveys were defined by a decision node (wait, reduce/space, adjust biologic). The items of the surveys, i.e., the pairs of scenarios, were created automatically by the programme after feeding it with all the factors and their corresponding levels. Impossible scenarios were deleted with the help of an expert (SM). The surveys were then sent to the panel of 17 experts.
Statistical analysisA conjoint analysis was used. This statistical technique makes possible to determine which combination of a limited number of attributes has the highest value in a decision12,13. The result of each selection of hypothetical scenarios is recorded as a rating contingent attached to attributes-levels in the scenarios presented, and an order from the most valued to the least valued (contingent ranking). The statistics, automatically calculated by the software used (1000Minds®), were the partial-value functions—i.e., the utility that physicians assign to the levels of each attribute—and the weights of relative importance—i.e., which attributes exert a considerable influence on choice. With these values, it is possible to define rankings, i.e., a value-ordered list of the factors that influence decisions, and weights, i.e., a measure of the magnitude of the influence. Rankings and weights are both valid ways to present the results, although they are not exchangeable; however, one can easily understand that between two magnitudes of influence, the largest will rank higher.
ResultsThe panel was composed by 6 men and 11 women, with ages between 32 and 65, being 8 paediatricians, 8 rheumatologists who attended adults and children, and 1 who had both specialities and attended only children currently. All were actively practicing in university hospitals and had access to transition policies and procedures. As part of the criteria, they were all experts in the boards of rheumatology and/or paediatrics associations and had a minimum 5-year experience with biologics.
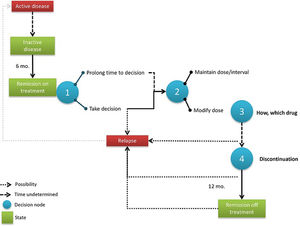
Process map and decision nodesFig. 3 shows the dose modification process map as discussed in the expert group with the three decision nodes identified. Experts agreed that the entire process begins when a patient arrives at “remission with treatment” for at least 6 months, a situation which is well defined by the Wallace criteria (Table 1).14
Definition of “remission on and off treatment”.
| Based on the Wallace criteria, a child or youngster with JIA is in remission if: |
| - No arthritis |
| - No fever, no rash, no serositis, no lymph nodes or myalgias attributable to the disease |
| - No active uveitis |
| - Normal acute phase reactants (PCR and VSG), or elevated by causes not attributable to JIA |
| - Morning stiffness<15min |
| - No activity by any scale used by the professional |
| Four possible situations: |
| 1. Active disease |
| 2. Inactive disease (Wallace criteria are met). It can be with or without treatment. |
| 3. Disease in remission on treatmenta – Inactivity persists>=6 months. |
| 4. Disease in remission off treatment – Inactivity persists>=12 months after treatment discontinuation. |
The first node implies postponing the decision (for an undetermined time) or not; the second decision node would be to modify the dose/interval or not; and the third decision node would be how to modify therapy. A fourth decision was identified in the longer term, when a patient achieves 12 months of remission with optimised treatment, but it was agreed by the panel that this decision was far too complex for this initiative given that there are many modification options available.
Factors determining decisionsIn decision node 1 (“Postpone decision to optimise”), and after the two rounds of consultation (small and broader group of experts), the following factors were identified: systemic or polyarticular predominance, macrophage activation syndrome (MAS) in the past, requirement of two or more treatments to be controlled or previous relapse while lowering the dose, time needed to achieve remission, subclinical inflammation (laboratory or imaging), involvement of joints difficult to control, tenosynovitis, uveitis, early debut (below the age of four), and axial involvement. Experts agreed in that these factors did not apply equally to all types of JIA (Table 2). The panel of experts established in the first meeting, by consensus, that the results should apply to the different JIA subtypes, grouped for this exercise purposes into: “systemic”, “polyarticular RF+”, “oligoarticular and polyarticular RF negative”, “arthritis-related enthesitis” and “psoriatic arthritis”. This information was used to prepare the scenarios and delete the impossible ones.
Factors that may lead to postponing the decision to modify dose. As elicited from experts discussions of experiences, by type of JIA.
| Attributes | Classification | |||
|---|---|---|---|---|
| Systemic | Polyarticular RF+ | Oligo-polyarticular RF− or psoriatic | Enthesitic-related arthritis | |
| Systemic predominance | X | |||
| Polyarticular predominance | X | |||
| MAS | X | |||
| Has required 2 or more treatments or has previously relapsed while lowering the dose | X | X | X | X |
| Time to remission | X | X | X | X |
| Subclinical inflammation (laboratory) | X | X | ||
| Subclinical inflammation (imaging) | X | X | X | X |
| Difficult to control joints | X | X | X | X |
| Tenosynovitis | X | X | X | |
| Uveitis | X | |||
| Early debut (age<4) | X | |||
| Axial involvement | X | |||
In the second decision node (“maintain or modify”) the factors affecting the decision are not so clearly related to patient attributes but to the drug, e.g., whether the drug is given in monotherapy or has produced immunogenicity.
In the third node (“how”), the experts identified the factors affecting the choice of drug to reduce, biologic or non-biologic. This node occurs immediately after the second node, or simultaneously. The factors affecting this decision that were elicited from the discussions were immunogenicity, adherence, and MTX tolerance. Further structure for the “how to” decision is presented in a supplementary file, as it was decided not to include given its complexity.
At the second meeting, the broader group ratified the previous attributes, added a few and defined each attribute and the levels of each factor (final list used in Table 3).
Factors and levels tested in the discrete choice experiment.
| Node | Factors/attributes | Levels |
|---|---|---|
| 1st | Systemic involvementa | Present |
| Absent | ||
| No. joints | <4 | |
| >=4 | ||
| Rheumatoid factor | Negative | |
| Positive | ||
| MASb | Has presented it in the past | |
| Has not presented it | ||
| No. treatments needed to reach remission | 1st DMARD | |
| 1 DMARD then 1st biologic | ||
| 2 or more biologics | ||
| Time to remissionc | <1 year | |
| >=1 year | ||
| Subclinical inflammation (laboratory)d | Present | |
| Absent | ||
| Subclinical inflammation (imaging) | Normal ultrasound, including Power-Doppler | |
| Power-Doppler signal in joint or tendon | ||
| Joints difficult to control | Temporomandibular joint | |
| Carpal synovitis | ||
| Tarsal-sub astragalin | ||
| Neck | ||
| Hips | ||
| Carpal tenosynovitis | ||
| Uveitise | <2 episodes, without complications | |
| >=2 episodes, without complications | ||
| With complications | ||
| Early debut (<4 years old) | Onset <4/onset >=4 | |
| Axial involvementf | Axial involvement/no axial involvement | |
| 2nd | Monotherapyg | Monotherapy/combined therapy |
| Immunogenicityh | Unknown drug levels | |
| Undetectable | ||
| Anti-drug antibodies | ||
| 3rd | Adherence | Poor/good |
| Methotrexate intolerance | Present | |
| Absent | ||
Table 4 shows the criterion ranking of the factors involved in the first decision node. To interpret it, the greater the ranking (1–14), the less difficulty or doubt to make the decision.
The participants mentioned that the presence of uveitis did not clarify the scenario and that they would have needed to know more about the specific case, such as whether the episode occurred while on treatment, or treatment required to control uveitis (topical, systemic, etc.).
In node 2 (“to modify or not”) the two attributes investigated showed a very similar weight (in this case the ranking had less meaning as there were only two attributes), although the presence of antidrug antibodies made the choice of modifying simpler (weight 6) than if the patient is with more than one drug (combined therapy) for the control of the disease (weight 4). It was mentioned that it may depend on the biologic agent used, as not all have the same antibody profile.
In node 3 (“which drug regimen to modify and how”), the same situation occurred, the attributes had a similar weight, although the presence of previous toxicity with MTX made the choice simpler (weight 6) than low adherence (weight 4).
An analysis of attributes by specialty showed no differences or patterns between the preferences of paediatricians (who practice rheumatology) and rheumatologists (who attend children).
DiscussionResults show that, for experts in the use of biologics in JIA, the decision is clear when the patient has had a MAS, or systemic involvement, or subclinical inflammation, either analytical or by image, even if at present the patient has been on remission for the last 6 months. The preferred decision was, in all these cases, to postpone or to avoid optimisation. Interestingly enough, other aspects identified by experts as modifying their decisions were not really influencing it, especially the involvement of joints difficult to control or age at debut. Other aspects had different effect when confronted to experts as part of hypothetical scenarios.
The vast majority of randomised controlled trials of biological therapies in children have a phase in which withdrawal is implemented once remission is achieved.7,15–20 This reflects common practice in paediatric rheumatology, where medications are discontinued as soon as possible to avoid long-term complications. This has led to an “art of optimisation”, with each paediatric rheumatologist taking different decisions or using different factors to support them. While variability is not per se unwanted, when it exists, it should be based on solid grounds of proven efficacious and safe options.1 As Halyabar et al. nicely summarised in their systematic review, the disease is heterogeneous, the definition of inactivity is heterogeneous, disease management is heterogeneous, consequently, “decisions often revolve around perceptions of the relative risks of continuing versus stopping treatment”.5
Several studies, many small, most retrospective, have studied outcomes of children with JIA who discontinued therapies after achieving inactive disease.1,5–7 Evidence shows that flares are common after discontinuation, ranging from 30 to 100%.5 This is one of the reasons why optimisation rather than withdrawal is attempted.2,7,21 However, optimisation is not as well studied as withdrawal, neither are factors influencing outcome, and paediatric rheumatologists do not have a guidance on how to actually do it with guarantees in JIA, as it is the case with adults.4
We are confident in that the results reflect actual mental processes based on experience. The discrete-choice method interrogates highly experienced individuals and confront them with a set of hypothetical scenarios with several levels of two or more attributes and ask them to choose the one they prefer (choice experiment).13 This method identifies preferences and factors influencing decisions more efficiently than real data, at least with feasible numbers and wealth of factors. This methodology also has some drawbacks. Respondents use simplification strategies when facing many options, when the reality is that the situation in front of a patient is far more complex than two variables at a time.13 Experts would have wanted more information and more options, as shown by their comments on uveitis, for example. Also, the experts responding the survey could have used simplification strategies, for instance, always choosing conservative options, a limitation we cannot rule out with this design. However, our intention was to identify the factors that most plausibly influence the decision and to rank them, rather than obtaining a composite weighted score or similar output. We cannot discard that, in the future, the understanding of decisions could be better carried out with some method using artificial intelligence.22 Currently, this is the most adequate methodology we could think of to help guiding decisions of non-experts.
In our initiative, we have attempted to study the decision process by first having experts discussing on their experiences and studies, then by testing the factors they think they use in their decisions to weight the importance in the decision. This experiment found no sociodemographic factors determining decisions, while in many studies these are the first factors to study,4,6,7,23 and identified other factors that should now be tested or used for studies in the long term. The discussions were extremely rich and factors that appeared initially as determinants, then faded away when seen on light of other factors, e.g., comorbidity, like psoriasis or inflammatory bowel disease were put forward as factors in a first step, but when the classification into four groups was established as well as the starting point of remission, they disappeared as relevant for the decision to taper. It could be argued that the number of experts was low and that the survey should have had many more respondents. Given the complexity of the clinical decision making in this specific situation, our resolution was to weight expertise more than sample size, as the final aim was to use these results to inform recommendations on the adjustment of biologics in JIA. Also, not including experts from other countries was determined to facilitate discussions in a common language in which all were fluid, as our experience is that Spanish experts are excellent clinicians but do not participate comfortably in international panels due to language constrictions.
The next steps will be to study the appropriateness of each optimisation strategy (continuation of decision node 3), but this will require a different approach. Our aim for a second step is to review the literature on available options on how to taper biologics, and to run a national survey, targeted at paediatric rheumatologists, on tapering practices. Ideally, we should design large observational studies to compare the different options, but this is not easy in the context of rare diseases.
In summary, the decision to optimise the dose of biological therapy in children and youngsters can be granulated into several nodes, and the factors, both patient and therapy-related, leading to the decision can be detailed. These decisions taken by experts may be transported to practice, study designs, and guidelines.
Data sharing statementData are available upon request.
Authors’ contributionsSM designed, coordinated, analysed and wrote the manuscript; AB, IC, EN, BB, SB, MC, DC, JG, JdI, LLC, PMC, JCNG, MCP, EQ, CV, and JA contributed to the discussions, answered the discrete choice surveys and helped in the preparation of the manuscript. All authors revised the last version.
Ethical approval and patient and public involvementThe nature of the study, theoretical and with no inclusion of real patients’ data, does not make it necessary to undergo ethical review according to local regulations. For its technical difficulty and given that all decisions are physician based, patients or public were not involved in the project.
FundingThe study was funded by the Spanish Society of Paediatric Rheumatology (SERPE).
Conflicts of interestS.M reports grants from Spanish Paediatric Rheumatology Society; A.B has nothing to disclose; I.C.P has nothing to disclose; E.N.C reports grants from Spanish Paediatric Rheumatology Society; B.B reports grants from SERPE; S.B has nothing to disclose; M.C.L reports grants from SERPE; D.C. reports grants from Spanish Paediatric Rheumatology Society, from null, during the conduct of the study; personal fees from Lectures for Abbvie, Roche and Novartis, outside the submitted work; B.B reports grants from SERPE; J de I. reports grants from Spanish Paediatric Rheumatology Society; L.L. has nothing to disclose; P.M.D.C. has nothing to disclose; J.C.N.G. has nothing to disclose; MC.P. reports grants from SERPE; E.Q-M has nothing to disclose; C.V. reports other from Pfizer, Abbie, Roche, Novartis, UCB, SERPE, SER, FISEVI; J.A. reports grants from Spanish Paediatric Rheumatology Society.
The authors want to thank InMusc, especially Estibaliz Loza and Loreto Carmona for facilitating the meetings, preparing the surveys and editing the manuscript.