SYSADOAs (Symptomatic Slow-Acting Drugs for Osteoarthritis) are natural compounds that have been shown to be useful and safe in the treatment of osteoarthritis (OA). However, their use in certain clinical situations still lacks scientific evidence and clear recommendations. The objective of this work was to learn the opinion of a group of experts regarding the appropriate use of SYSADOA in the treatment of OA in controversial clinical situations.
Materials and methodsFollowing the Delphi technique, 206 specific consultations, structured in 24 clinical questions, were evaluated. A panel of experts composed of a total of 15 specialists, answered the two rounds of consultation through an online platform. The results were analysed and discussed in a face-to-face meeting with the coordinators and the scientific committee. According to the percentage of panellists who agreed on their findings, the results were classified in terms of unanimity, consensus, majority and discrepancy.
ResultsThe following points were agreed upon: (1) the patient's phenotype determines the use of SYSADOAs; (2) SYSADOAs are considered appropriate in primary OA (knee, hand and hip) and in some types of secondary OA; they are not considered appropriate in OA of the shoulder, spine, ankle and erosive OA of the hands; (3) SYSADOAs may be prescribed for patients at risk of or with cardiovascular disease, digestive disease, hypertension, dyslipaemia, peripheral vascular disease, type 2 diabetes and, excluding Diacerein, for patients with oesophageal reflux. No agreement was obtained on the prescription of SYSADOAs for patients with hepatic and renal disease.
ConclusionsThere is limited literature on the use of SYSADOAs for the treatment of OA in controversial situations. Through this work it has been possible to establish the position of a group of experts regarding clinical situations for which there is no scientific evidence concerning their use. This work may contribute towards improving the management protocols of SYSADOAs in the treatment of OA and offer a useful approach in uncertain situations.
Los SYSADOA (del inglés, Symptomatic Slow-Acting Drugs for OsteoArthritis) orales son compuestos naturales que han demostrado ser útiles y seguros en el tratamiento de la artrosis (AO). Sin embargo, su uso en ciertas situaciones clínicas carece aún de evidencia científica y recomendaciones claras. El objetivo de este trabajo fue conocer la opinión de un grupo de expertos sobre uso de los SYSADOA en el tratamiento de la AO en situaciones clínicas controvertidas.
Materiales y métodosSiguiendo el método del uso apropiado mediante la técnica Delphi, se valoraron 206 consultas concretas, estructuradas en 24 preguntas clínicas. Un panel de expertos, compuesto por un total de 15 especialistas, respondió a las dos rondas de consulta a través de una plataforma online. Los resultados se analizaron y debatieron en una reunión presencial con los coordinadores y el comité científico. Según el porcentaje de panelistas que coincidieron en los mismos, se clasificaron los resultados en términos de unanimidad, consenso, mayoría y discrepancia.
ResultadosSe consensuaron los siguientes puntos: (1) el fenotipo del paciente condiciona el uso de los SYSADOA orales; (2) los SYSADOA orales se consideran adecuados en la AO primaria (rodilla, mano y cadera) y en algunos tipos de AO secundaria; no se consideran adecuados en AO erosiva de manos, hombro, columna y tobillo; (3) los SYSADOA orales pueden ser prescritos a pacientes con riesgo o enfermedad cardiovascular, enfermedad digestiva, hipertensión, dislipemia, enfermedad vascular periférica, diabetes tipo 2 y, a excepción de Diacereína, en pacientes con reflujo esofágico. No se obtuvo acuerdo en la prescripción de los SYSADOA orales en pacientes con enfermedad hepática y renal.
ConclusionesExiste escasa literatura sobre el uso de los SYSADOA orales en el tratamiento de la AO en situaciones controvertidas. Mediante este trabajo se ha podido conocer el posicionamiento de un grupo de expertos frente a situaciones clínicas para las cuales no existe evidencia científica respecto a su uso. Este trabajo puede contribuir a mejorar los protocolos de manejo de los SYSADOA en el tratamiento de la AO y ser un instrumento útil en situaciones de incertidumbre.
Osteoarthritis (OA) is a process characterised by the degradation and loss of joint cartilage with varying degrees of local inflammation, preferentially affecting load-bearing joints. Clinically, it is primarily associated with joint pain and stiffness, and is the most common cause of chronic pain and disability in older people.1 The EPISER study, conducted by the Spanish Society of Rheumatology, found that 13.9%, 7.9% and 5.2% of the population aged 20 years or older living in Spain had symptomatic OA of the knee, hand, and hip, respectively.2
OA is a major socioeconomic problem. In Spain, the ArtRoCad study attributes an average annual cost of 1502 Euros per patient to knee and hip OA, which amounts to a total cost of 4738 million Euros per year.3 This figure is in line with most Western countries.4
Traditionally, the disease has been treated purely symptomatically with analgesics and non-steroidal anti-inflammatory drugs (NSAIDs). However, the pharmacopoeia available is sparse and not without its problems. The various meta-analyses establish a therapeutic effect for paracetamol below the threshold of clinical significance.5 Chronic use of NSAIDs in older adults has significant cardiovascular, gastrointestinal, hepatic and renal side-effects.5 The pain-relieving action of opioids is counteracted by their side effects, especially in older patients.5,6 Finally, the intra-articular administration of drugs (corticosteroids and hyaluronic acid) causes discomfort for patients, and the risk of infectious arthritis associated with their use is not negligible.5
Another group of drugs for the treatment of OA are the SYSADOA (symptomatic slow-acting drugs for osteoarthritis), which are natural, structurally heterogeneous compounds. Chondroitin sulphate (CS), glucosamine (G), the two in combination (CS/G) and diacerein (D), hereafter oral SYSADOA, have been shown to reduce pain and stiffness and increase functional capacity, while generally ensuring a good safety profile.7–9 However, inconsistent results observed in certain studies and meta-analyses have generated debate on their use.10,11 In fact, not all patients respond to oral SYSADOA,9,10 and evidence on their use in certain patient profiles and clinical situations is still limited or non-existent.
The inconsistent results and lack of evidence in certain clinical situations have resulted in different recommendations in the various therapeutic guidelines.5,12–14 Heterogeneity in the recommendations for the use of oral SYSADOA and, consequently, in prescribing by specialists, may result in inappropriate use.
In this context, the main objectives of this study were to obtain the opinion of a group of experts on the use of SYSADOA in controversial clinical situations or where there is no evidence to support their use, and to provide guidelines for their appropriate use aimed at the different professionals treating this disease.
Materials and methodsIn this work promoted by the Osteoarthritis Foundation International (OAFI), we used the modified appropriateness method, using the two-round Delphi technique. This method, developed by researchers at the RAND Corporation and the University of California, Los Angeles (UCLA), is based on the available scientific evidence and the opinion of a panel of experts.15
Reading of the literature and drafting of the document’s indexThe project coordinators (a clinical coordinator specialised in rheumatology and a methodological coordinator specialised in clinical pharmacology) compiled the relevant literature on the use of SYSADOA. The clinical situations to be addressed in the Delphi questionnaire were defined based on the thematic index that was developed (Table 1).
Document index.
| Perception of the clinical-therapeutic usefulness of oral SYSADOA |
| 1. General use of oral SYSADOA according to risk factors presented by the patient |
| 2. General use of oral SYSADOA according to type of OA |
| 3. General use of oral SYSADOA according to the patient’s level of pain |
| Evidence for oral SYSADOA in the context of the treatment of OA |
| 4. Evidence for the efficacy of treatment with oral SYSADOA in OA |
| 5. Experience in the time from prescription of oral SYSADOA to reaching clinical-therapeutic effect |
| 6. Experience in the time from prescription of oral SYSADOA until achieving maximum therapeutic efficacy |
| 7. Experience in continuing treatment with oral SYSADOA after an initial positive response |
| 8. Experience in considering the non-efficacy of oral SYSADOA |
| 9. Experience in increasing the dose of oral SYSADOA |
| 10. Experience with persistence of effect of oral SYSADOA after discontinuation |
| 11. Dosage and treatment regimen of oral SYSADOA |
| Appropriateness of oral SYSADOA in the therapeutic setting of OA and its comorbidities |
| 12. Appropriateness of oral SYSADOA in secondary OA |
| 13. Appropriateness of oral SYSADOA in patients with prostheses |
| 14. Appropriateness of oral SYSADOA in comorbid and polymedicated patients |
OA: Osteoarthritis; SYSADOA: Symptomatic Slow-acting Drugs for Osteoarthritis.
The Delphi questionnaire included a total of 24 clinical questions and 206 outcomes (each of the variables covered in the same clinical question). The questionnaire included clinical situations for which, to date, there is evidence to support the use of SYSADOA and other situations for which there is insufficient evidence. These issues that can create uncertainty and inappropriate use were finally analysed. The final questionnaire was elaborated through a face-to-face meeting involving the coordinators and a scientific committee (SC), composed of 2 rheumatology specialists and 2 primary care (PC) physicians.
Selection of the expert panelThe panel members who answered the questionnaire were selected jointly by the coordinators and the SC. The main selection criteria were: 1) the representation of all specialties involved in OA management and the representation of a clinical pharmacology specialist, 2) OA as the main area of interest and 3) geographical diversity. For the clinical specialists, criterion 4) 10 or more years of clinical experience in the use of oral SYSADOA was specifically added; and for the clinical pharmacology specialist, two criteria were added, 5) published studies reflecting expertise in drug titration and in the field of inflammation and/or OA, and 6) professional activity linked to a care centre. The identity of the panellists was masked throughout the process to prevent any interaction between them.
Profile of the panellistsSixteen professionals were invited to form the expert panel, 15 of whom accepted and answered the two rounds of consultation. According to the defined selection criteria, there were 10 PC physicians, one rheumatologist, one traumatologist, one rehabilitation specialist, one gynaecologist and one clinical pharmacologist. The geographical distribution by region was as follows: 4 panellists from Catalonia, 4 from Madrid, 3 from Galicia, one from Andalusia, one from Castilla-La Mancha, one from Castilla y León, and one from the Valencian Community.
Consensus criteriaTo interpret the results, it was agreed that the responses would be grouped according to the percentage of panellists who agreed on the same answer with respect to the total, as follows: unanimity (when 100% of the panellists agree), consensus (when <100% and ≥80% agree), majority (when <80% and ≥66% agree) and discrepancy (when <66% agree).
Consultation roundsFirst roundEach panellist received by email the link to the online platform, personal login credentials and the response deadline for the first round. The platform contained the questions of the questionnaire, a brief introduction prior to each consultation to contextualise, and the answer type (yes/no, Likert scale 1–4 or single response). Once this first round had been completed, the coordinators and SC analysed the results. After this first analysis it was considered that the wording was not sufficiently precise and could lead to confusion, therefore we decided to modify the wording or answer type for a total of 25 outcomes. One outcome was removed, and 21 new outcomes were added. When the discrepancy was not due to imprecise wording, but to lack of agreement between the panellists due to different clinical judgements, a new comment box was added to allow the panellists to argue their position.
Second roundIn the second round, the panellists responded only to the outcomes not agreed in the first round, with the possibility of viewing their answer for each outcome in the first round and the overall position of the other panel members.
Analysis of responsesAnalysis was performed by consulting and exploiting the answers present on the database, from the online platform. The number of panellists who agreed on the same answer was analysed; the values were presented as "n" and percentage. Based on the answer with the highest percentage, the result was established in terms of unanimity, consensus, majority, or discrepancy.
Final meeting and drafting of the reportThe results obtained after the two rounds of consultation were sent to the coordinators and SC for assessment and discussed in a face-to-face meeting.
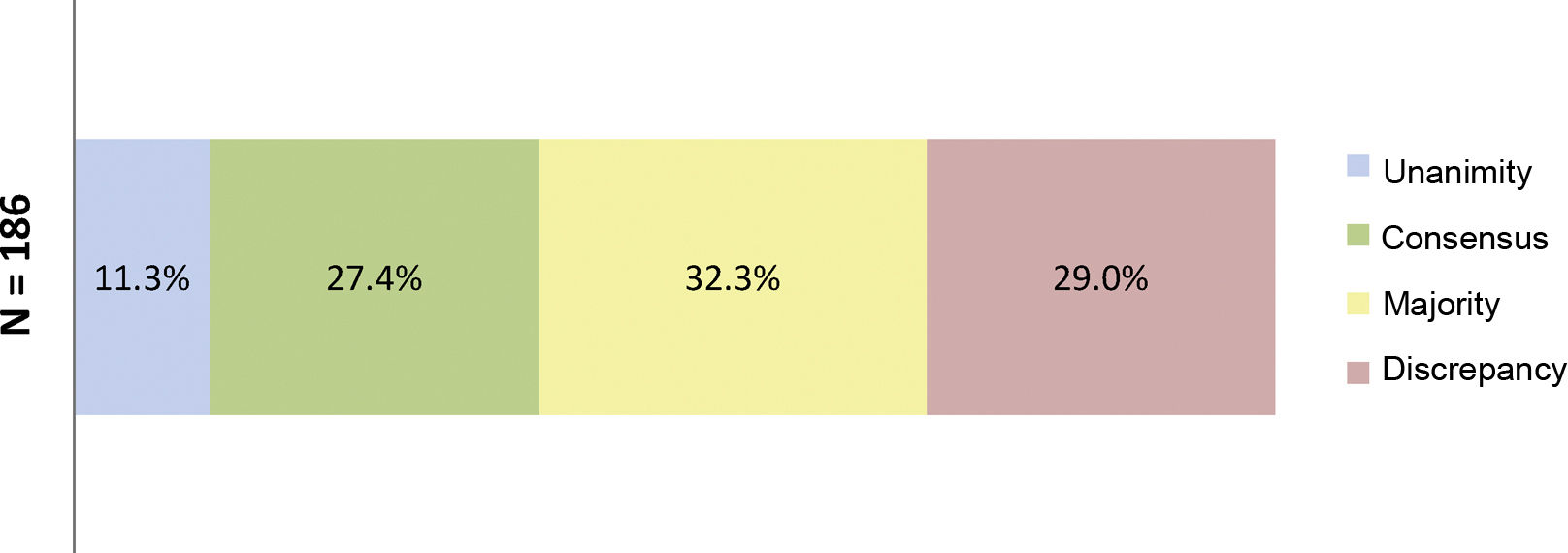
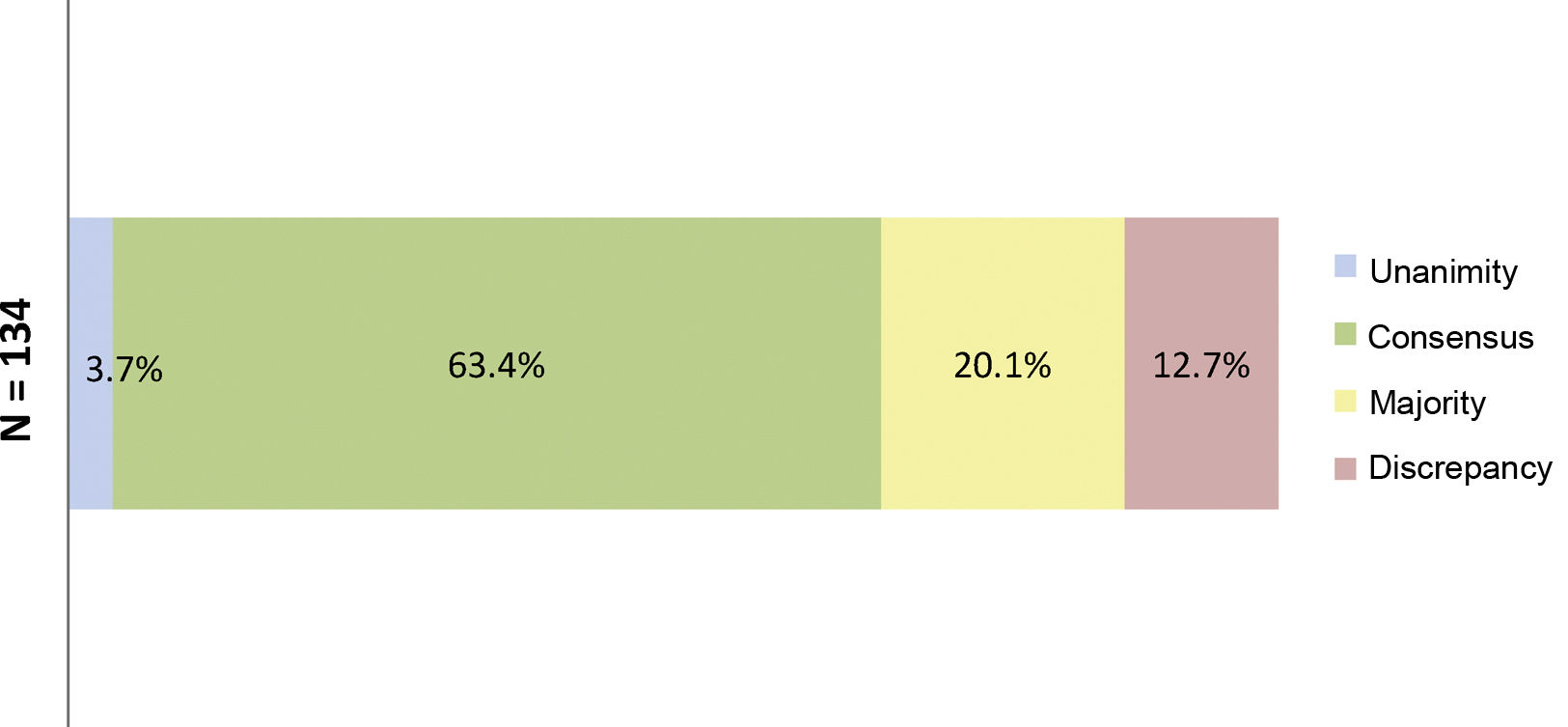
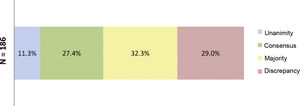
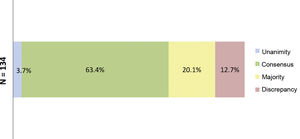
ResultsResults of the Delphi questionnaireA total of 186 outcomes were included in the first round; 38.7% were agreed (11.3% unanimously) and 29% disagreed (Fig. 1). In the second round, 134 outcomes were analysed (114 that achieved majority and discrepancy results in the first round, 21 new outcomes and one was eliminated); 67.3% of the outcomes were agreed (3.7% unanimously) and there was discrepancy in 12.7% (Fig. 2).
Percentages obtained after completion of the first consultation round.
Fig. 1 shows the percentages obtained in terms of unanimity, consensus, majority, and discrepancy after completion of the first Delphi round.
N = total number of outcomes consulted in the first round.
Percentages obtained after completion of the second consultation round.
Fig. 2 shows the percentages obtained in terms of unanimity, consensus, majority, and discrepancy after completion of the second Delphi round.
N = total number of outcomes consulted in the second round.
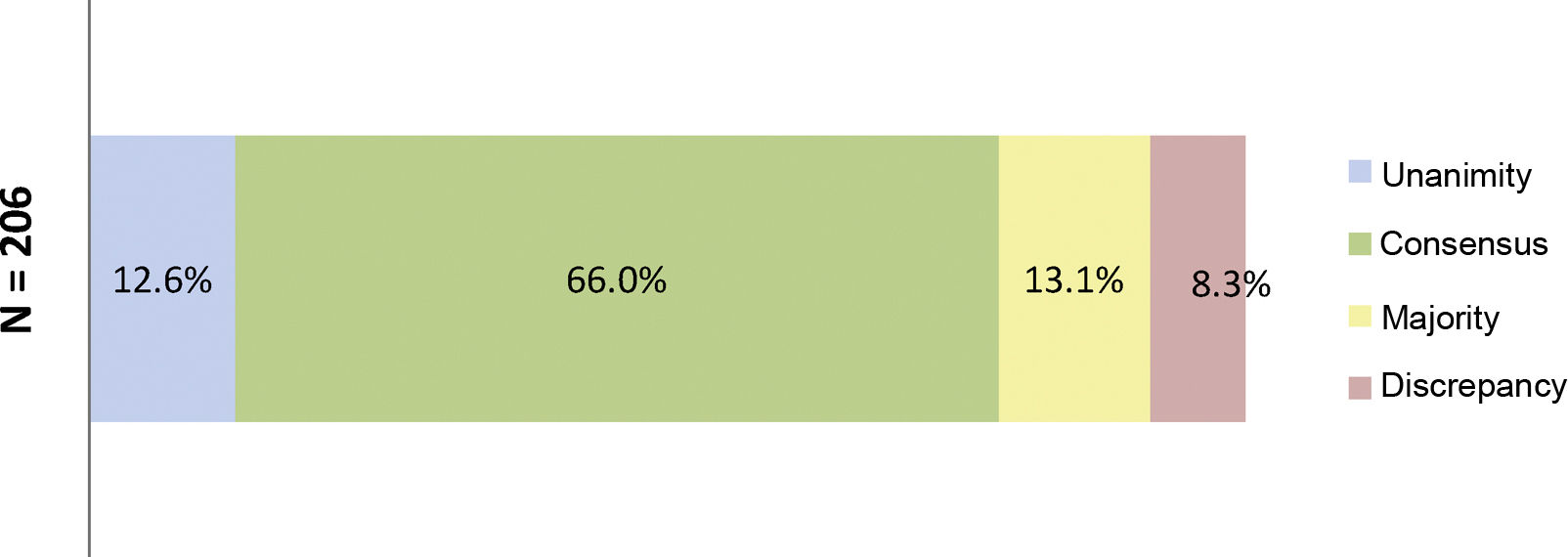
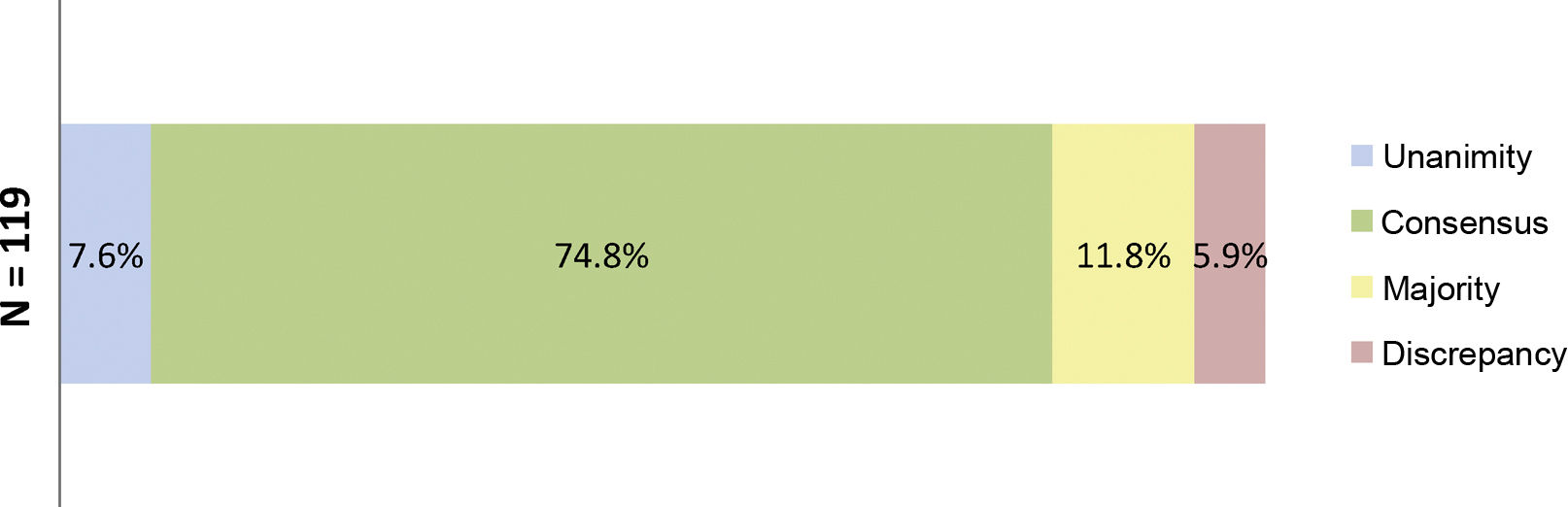
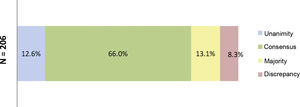
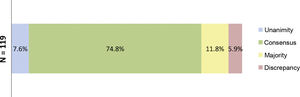
At the end of the two rounds of consultation, a total of 206 outcomes had been reviewed (72 outcomes agreed in the first round plus the 134 outcomes included in the second round). Unanimity (12.6%) was obtained for 26 outcomes; consensus (66.0%) for 136 outcomes; majority (13.1%) for 27 outcomes; and discrepancy (8.3%) for 17 outcomes (Fig. 3). The aspects were analysed where there was contention about SYSADOA, concerning efficacy according to risk factors and location of disease, dosage and treatment regimens and the safety of the drugs analysed (Fig. 4).
Percentages obtained after the two consultation rounds.
Fig. 3 shows the percentages obtained in terms of unanimity, consensus, majority, and discrepancy after completion of the second Delphi round.
N = total number of outcomes consulted at the end of the study.
Percentages obtained after the two rounds of consultation of the aspects where SYSADOAs generate controversy.
Fig. 4 shows the percentages obtained in terms of unanimity, consensus, majority, and discrepancy at the end of the study.
N = total number of outcomes related to controversial clinical situations or without evidence to support the use of oral SYSADOA.
The panellists agreed on the use of oral SYSADOA in primary OA in the first three Kellgren and Lawrence16 radiological classification grades and in the three subsets of the Herrero-Beaumont classification criteria.17
The expert panel considered the use of SYSADOA appropriate in primary internal and external femorotibial knee osteoarthritis, hand OA (proximal interphalangeal and distal interphalangeal joints), patellofemoral OA, rhizarthrosis, hip OA, and secondary traumatic OA and with alignment disorders. Use of oral SYSADOA in primary shoulder, spine, ankle, or erosive hand OA, as well as in secondary microcrystalline OA or with underlying immune-mediated disease, was considered inappropriate (Appendix B Supplementary Table 1).
Phenotypic characteristicsThe panellists agreed that certain modifiable risk factors, such as strenuous and/or repetitive housework, previous trauma, or previous trauma surgery, influence the use of oral SYSADOA.
Other risk factors not covered in guidelines or trials, such as overweight, age under 40 years and gender in patients under 40 years of age, were also considered determinants for the use of oral SYSADOA (Appendix B Supplementary Table 1).
Treatment dose and regimenThe expert panel agreed that the therapeutic (and starting) doses of CS and G should be 800 mg and 1500 mg, respectively, as indicated in their data sheets (DS). No consensus was reached, however, on what the therapeutic dose of CS/G should be: most of the experts believed it should be 800 mg CS + 1500 mg G. There were discrepancies regarding the therapeutic dose of D.
Most of the panellists agreed to include 2-month rest intervals in the CS treatment regimen. There was no agreement on the treatment regimen for the other three oral SYSADOA (Appendix B Supplementary Table 2).
SafetyIn the present study, most of the panellists believed the prescription of oral SYSADOA is appropriate in patients with the following comorbidities: cardiovascular disease or risk, digestive disease, hypertension, dyslipidaemia, peripheral vascular disease, type 2 diabetes, and oesophageal reflux. They considered the use of D inappropriate in patients with oesophageal reflux.
The expert panel did not consider it appropriate to prescribe these drugs in patients with liver or kidney disease, irrespective of the extent of the disease. Discrepancies were noted regarding the use of CS in patients with liver disease. The experts also disagreed with prescribing oral SYSADOA in patients with mild kidney disease (glomerular filtration rate 60−89 ml/min/1.73 m²).
There was consensus on the use of SYSADOA in patients requiring treatment with opioids, NSAIDs, anxiolytics or antidepressants. Most of the panellists disagreed with the use of SYSADOA in patients with lactose, sorbitol, or shellfish allergy (Appendix B Supplementary Table 3).
DiscussionThere is scientific evidence to support the use of oral SYSADOA in certain clinical situations. However, prescribing physicians do not always make decisions based on the evidence and therapeutic guidelines available to them; they use their judgement based on experience. There are other clinical situations in which the use of SYSADOA is not supported by scientific evidence or is disputed. The present study provided the opinion of a group of experts regarding routine clinical practice in situations where there is insufficient evidence.
There is evidence on the efficacy of SYSADOA for the treatment of Kellgren and Lawrence grades II–III knee OA affecting the internal femorotibial compartment and in OA of the hand.7,8 However, few articles have been published on the efficacy of SYSADOA in the other knee compartments or the trapeziometacarpal joint.7,18,19 In this case the expert panel recommended the use of SYSADOA for any joint of the knee and hands. In OA locations where there are no references in the literature the experts agreed that SYSADOA should not be used. The experts' opinion was probably influenced by the fact that this is a chronic disease that generally affects older adults for which the therapeutic offer is limited and incomplete.
Without having fully contrasted scientific evidence but in line with the recommendations published in the SEMERGEN guidelines20 for the treatment of OA, the panellists agreed that risk factors such as repeated overloads, previous trauma, and surgery, overweight and age are determinants when prescribing this group of drugs.
The group of experts mentioned that the doses of G and CS are 1500 and 800 mg/24 h, respectively, when prescribed separately or in combination. In this case the experts relied on each drug’s separate DS, as opposed to the DS of the combined drugs and the GAIT study where 1200 mg of CS and 1500 mg of G10 were used. SYSADOA have been demonstrated to interfere with the natural progression of the disease. In this sense, different practitioners might pursue different therapeutic goals, which would result in the use of doses other than those recommended for the symptomatic treatment of OA. There were also discrepancies regarding the therapeutic dose of D, even though the DS indicates 100 mg/day. These discrepancies could be partly due to the specialists' caution in increasing the dose from 50 mg/day (initial) to 100 mg/day (therapeutic), due to possible side effects, especially intestinal side effects.21–23
The panellists opted to recommend 2 months of rest after CS treatment as recommended by its DS. A plausible explanation for this agreement by the experts is the known carry-over effect of the drug.24 For the other SYSADOA the lack of agreement on treatment regimen is probably because it is not specified in the DS of G, D or CS/G.
All the above highlights the need to establish a homogeneous guideline for the use of oral SYSADOA that can be applied in routine clinical practice.
Oral SYSADOA are well-tolerated drugs with no significant side effects and no significant interactions, which are important requirements in the treatment of chronic diseases.20 Overall, the expert panel believed SYSADOA are safe drugs and can be prescribed in the presence of any comorbidity, except for D in underlying gastrointestinal diseases.
They did not consider it appropriate to prescribe SYSADOA in patients with underlying liver and kidney disease either, irrespective of the extent of the disease, given the lack of evidence in this regard. Discrepancies between each product’s DS, as well as the lack of specific studies, could explain the position of the panel members.25
Limitations of this studyExhaustive reading and selection of the literature was carried out for this work, although it can in no way be interpreted as a systematic review of the literature. Given the nature of the literature review, the outcomes shown are not accompanied by a level of evidence. The results of the present work are exclusively qualitative in nature, as the Delphi methodology only validates consensus among participants. A specific group of experts was selected to assess the outcomes, and therefore the sample might not be representative of the overall number of physicians involved in the management of OA.
ConclusionsIn the present study, a group of experts was consulted on the use of SYSADOA in the treatment of OA and consensus was reached on 78.6% of the outcomes surveyed, which enabled us to learn the position of the experts in clinical situations where there is no evidence or where it is contentious.
This is the first study to use the method of “appropriateness” to address controversial aspects of the use of oral SYSADOA. The results obtained highlight the need to establish new agreements as a useful tool in the management of patients with OA, especially in the PC setting.
FundingOAFI is a non-profit organisation that finances part of its research and social work from donations, grants and private funding from collaborations with supportive companies that adhere to high quality standards in the design, production, and distribution of their products.
Conflict of interestsThe authors have the following conflicts of interests to declare:
Jordi Monfort has no conflict of interests to declare.
Xavier Carné has no conflict of interest to declare.
Benjamín Abarca declares that he has given presentations at the SEMERGEN seminar and conference and participated in the PICASSO research project promoted by Bioiberica.
Sergio Giménez declares that he has given presentations at the SEMERGEN conference promoted by Bioiberica, in Meranani’s ReumAPtopics and in the Grünenthal pain congresses.
Montserrat Romera has no conflict of interests to declare.
Ingrid Möller declares that she has given talks for Bioiberica and participated in the PICASSO study research project, promoted by Bioiberica, as a researcher and in a clinical trial with hyaluronic acid.
Marco Bibas has no conflict of interest to declare.
Marianna Vitaloni has no conflict of interest to declare.
Aina Batlle has no conflict of interest to declare.
Josep Vergés has no conflict of interest to declare.
The authors would like to thank the following experts for their collaboration as panellists and in answering the two Delphi rounds: Dr Antonio García of the Instituto Fundación Teófilo Hernando, Dr Alba Gurt of the Centro de Salud Vila Olímpica (Parc Sanitari Pere Virgili), Dr Blanca Rodríguez-Borlado Díaz of the Centro de Salud Valdemoro, Dr Francisco Blanco of the Hospital Universitario de A Coruña, Dr Francisco Martínez García of the Centro de Salud Mansilla de las Mulas, Dr Jaume Claramunt of the Centro de Salud Morera-Pomar, Dr Juan Antonio Martín Jiménez of the Centro de Salud Buenavista, Dr José Caballero Vega of the Unidad de Gestión Clínica de Ogijares, Dr José Carlos Bastida Calvo of the Centro de Salud de Marín, Dr Joan Carles Monllau of the Hospital del Mar, Dr Juana Sánchez Jiménez of the Centro de Salud Daroca, Dr María del Pilar Rodríguez Ledo of the Hospital Universitario Lucus Augusti, Dr Rafael Balaguer of the Hospital Vithas-Nisa 9 de Octubre, Dr Santiago Palacios of the Instituto Palacios de Salud y Medicina de la Mujer and Dr Silvia Ramón of the Hospital Quirónsalud Barcelona.
They would also like to thank Dr Patrick du Souich for his critical review, OAFI volunteer Patrice Johnson for his support in the English translation of the abstract and GOC Networking for their participation as methodological advisor.
Please cite this article as: Monfort J, Carné X, Abarca B, Giménez S, Romera M, Möller I, et al. Documento de expertos sobre el uso apropiado de los SYSADOA en situaciones clínicas controvertidas. Reumatol Clin. 2021;17:595–600.