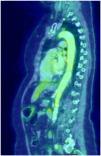
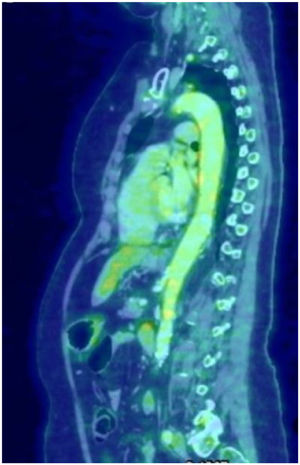
Giant cell arteritis (GCA) is a granulomatous vasculitis affecting the medium and large calibre arteries, most common in individuals over 50 years of age. The clinical spectrum is broad, ranging from classical manifestations such as headache, jaw claudication, visual disturbances, and polymyalgia rheumatica to various atypical manifestations. In 10%–15%, GCA affects the aorta and its branches; the aortic arch region and the thoracic aorta are the most affected areas; involvement of the entire aorta is uncommon1. On the other hand, aortic involvement has been described in more than 50% of the PET scans performed in patients with a confirmed diagnosis of GCA2. We present the case of a 69-year-old woman with no medical history who consulted due to a self-limited episode of left brachio-crural paresis, associated with a history of mandibular claudication for 15 days, asthenia, weight loss of 8 kg, and pain in the shoulder and pelvic girdle in the last month. Laboratory tests showed an increased erythrocyte sedimentation rate (ESR): 80 mm/first hour. Brain MRI, CT angiography of intracranial and extracranial vessels, echocardiography, and Doppler ultrasound of the temporal arteries showed no significant abnormalities. Because GCA was suspected, Fludeoxyglucose F18-PET (FDG-PET) imaging was requested (Fig. 1), which showed heterogeneous increase in metabolic activity in the walls of the aorta artery throughout its length, subclavian arteries, bilateral primitive carotid arteries and, to a lesser extent, bilateral primitive iliac arteries. The patient was initially treated with high doses of corticosteroids combined with methotrexate with poor response, and therefore tocilizumab was administered, showing improvement of symptoms and marked decrease in ESR. Patients with extracranial GCA often present with a non-specific clinical picture. For this reason, and due to the low sensitivity of temporal biopsy, diagnosis is usually made by PET, MRI, or angiotomography. The use of FDG-PET has facilitated evaluation of large vessel inflammation, with a high degree of sensitivity for detecting extracranial forms. It is the method of choice in patients with atypical symptoms, and has proved very useful for excluding other diseases such as malignancy3. The differential diagnosis is very broad, and it can be difficult to differentiate from elderly patients with Takayasu's arteritis. In this sense, FDG-PET can be useful since in GCA the distribution of the aortic lesion is diffuse and distal, with involvement of the abdominal aorta and its descending branches, and slight morphological changes of the vascular wall, whereas in Takayasu's arteritis the distribution is segmental and there are greater morphological changes4. However, several diseases may mimic large vessel vasculitis due to the presence of constitutional symptoms or due to the formation of aneurysms or stenoses in the aorta or its branches. All causes of aortitis must be excluded. Infectious causes include mycotic aneurysms, syphilis, and mycobacterial infections. In addition, inflammation of the aortic artery may occasionally occur in ANCA-associated vasculitis, Behçet's disease, ankylosing spondylitis, sarcoidosis, Sjögren's syndrome, and IgG4 disease. Atherosclerosis should always be considered in the differential diagnosis. Finally, aortitis can be isolated and idiopathic5. Complications are aneurysm or dissection primarily of the ascending aorta, aortic arch syndrome, limb claudication, and stroke1. Treatment strategies are the same as for patients with temporal arteritis6, although the disease tends to be more refractory7.
The Impact Factor measures the average number of citations received in a particular year by papers published in the journal during the two preceding years.
© Clarivate Analytics, Journal Citation Reports 2025
SRJ is a prestige metric based on the idea that not all citations are the same. SJR uses a similar algorithm as the Google page rank; it provides a quantitative and qualitative measure of the journal's impact.
See moreSNIP measures contextual citation impact by wighting citations based on the total number of citations in a subject field.
See more