Evaluate response to treatment and progression to rheumatoid arthritis (RA) in patients with fibromyalgia (FM) associated with elevated rheumatoid factor (RF).
Material and methodsProspective cohort study. The sample consisted of 124 patients with FM: 62 with high RF (>20 U/mL) and 62 with negative RF (0−20 U/mL). All patients were evaluated using FM treatment improvement score (FIQR) and progression to RA according to EULAR/ACR 2010 criteria at 6 and 12 months. Pearson's χ2 test for homogeneity was used to relate variables of improvement to FM treatment and progression to RA.
ResultsThe response to treatment was lower in the high RF group (24 and 20 patients improved at 6 and 12 months, respectively, compared to 45 and 38 patients in the negative RF group), with a significant difference. Progression to rheumatoid arthritis was similar in both groups (5 in the high RF group and 4 in the negative RF group), with a non-significant relationship.
ConclusionsFM with elevated RF is associated with a poor therapeutic response but not with progression to RA.
Evaluar respuesta a tratamiento y progresión a artritis reumatoide (AR) en pacientes con fibromialgia (FM) asociado a factor reumatoide (FR) elevado.
Material y métodosEstudio de cohortes prospectivo. La muestra se conformó por 124 pacientes con FM: 62 con FR elevado (>20 U/mL) y 62 con FR negativo (0−20 U/mL). Todos los pacientes fueron evaluados mediante una puntuación de mejora de tratamiento de FM (FIQR) y progresión a AR según criterios EULAR/ACR 2010 a los 6 y 12 meses. Se usó la prueba χ2 de Pearson de homogeneidad para relacionar variables de mejora al tratamiento de FM y progresión a AR.
ResultadosLa respuesta al tratamiento de FM fue menor en el grupo FR elevado (24 y 20 pacientes mejoraron a los 6 y 12 meses respectivamente, comparados con los 45 y 38 pacientes del grupo FR negativo), hallándose diferencia significativa. La progresión a artritis reumatoide fue similar en ambos grupos (5 en el grupo FR elevado y 4 en FR negativo) siendo una relación no significativa.
ConclusionesLa FM con FR elevado se asocia a mala respuesta terapéutica pero no con progresión a AR.
Fibromyalgia (FM) is a clinical syndrome the hallmarks of which are chronic generalised pain, joint stiffness, fatigue, sleep disturbances, and cognitive impairment, all in the absence of a systemic disease that would otherwise account for the condition. If we consider that the World Health Organisation (WHO) has declared chronic pain to be a public health problem, fibromyalgia can be declared to be a major concern around the world.1
The aetiology and pathogenesis of FM have yet to be fully established. Factors such as central and autonomic nervous system dysfunction, neurotransmitters, hormones, immune system, stressors, and psychiatric disorders have been associated with FM. Given the poor understanding of its pathogenesis, response to treatment has tended to be very poor in FM.2
Contrary to popular belief, arriving at a diagnosis of FM is a complex task, given that the symptoms are vague and generalised, and require that well-directed, additional testing be conducted; furthermore, there is no gold-standard test for diagnosing FM. It is clear that there is no cut-off point that is capable of distinguishing FM from non-FM. The diagnosis of FM is based primarily on the presence of two validated criteria, those of the 1990 and 2010 ACR.3,4
There is a group of people with FM who exhibit elevated levels of rheumatoid factor (RF) or antinuclear antibodies (ANA), without this necessarily denoting the presence of an autoimmune disease; however, the appearance of clinical signs suggestive of autoimmunity (fever, synovitis, skin involvement, neuropathy, muscle weakness, etc.) affects both diagnosis and treatment. Furthermore, a varying percentage of the world's population exhibits high levels of RF and ANA, without displaying any clinical signs whatsoever.5,6
FM treatment is currently being developed. In 2017, the European Alliance of Associations for Rheumatology (EULAR) recently put out their recommendations as to proper FM treatment. The drugs that achieved a IA level and grade of evidence were duloxetine, milnacipram, tramadol (with or without paracetamol), pregabalin (for severe pain), amitriptyline, cyclobenzaprine (for sleep disturbances). Sound judgement is advised when combining these drugs in order to achieve clinical remission in FM patients.7
There is a validated score for response to FM treatment, the Fibromyalgia Impact Questionnaire (FIQ).
It was first published in 1991 and has been widely used since then to evaluate treatment response in FM. In 2009, a modified FIQ (FIQR) was developed, which is both quicker and easier to administer than the original score. The FIQR consists of 21 questions structured into three domains: difficulty in carrying out a particular activity (9 items), impact on quality of life (2 items), and symptom intensity (10 items). Each question is assigned a score from 1 to 10. All of the scores for each item in each domain are added together. The summary score for domain 1 is divided by 3; domain 2 remains unchanged, and the total for domain 3 is divided by 2. The total is then totalled, and this is the final FIQR score, with a maximum score being 100. Values of between 50 and 70 are indefinite and should be interpreted according to the patient's condition.8
There is virtually no information available or research work undertaken concerning progression to RA or response to treatment in patients with FM and high RF. Consequently, we decided to conduct the present study, the primary objective of which was to evaluate treatment response and progression to rheumatoid arthritis (RA) in individuals suffering from fibromyalgia (FM) associated with high rheumatoid factor (RF), in an attempt to typify this class of subjects so as to be able to provide differential treatment and follow-up on the basis of our results.
Material and methodsA prospective, observational cohort study was performed. The sample consisted of 124 patients with a definitive diagnosis of FM according to 1990 or 2010 criteria and divided into two groups (the elevated RF group: 62 cases; the negative RF group: 62 patients). Response to FM treatment was assessed using the modified fibromyalgia impact questionnaire (FIQR) and progression to RA using the ACR 1987 or EULAR/ ACR 2010 criteria in both groups, at both 6 and 12 months. The first-line pharmacological treatment for FM (as recommended by EULAR 2017) used in this study were pregabalin, duloxetine, tramadol, milnacipram, amitriptyline, and cyclobenzaprine.
Follow up was 12 months. Inclusion criteria comprised having a diagnosis of primary FM (as per ACR 1990 or ACR 2010 criteria) with elevated RF, while exclusion criteria consisted of: secondary FM in connection with an underlying disorder, elevated anti-CCP antibodies, excellent response to corticosteroids, concurrent autoimmune disease, inflammatory polyarthropathy, pregnancy, co-existing chronic disease, and the presence of synovitis on physical examination or imaging study.
The operational definitions for this work were: (1) Patient with primary FM: patient meeting ACR 1990 or ACR 2010 criteria for FM, without inflammatory arthropathy or chronic disease, without synovitis, with positive or negative RF, anti-CCP within normal ranges. (2) Patient with RA: patient meeting ACR 1987 or EULAR/ACR 2010 criteria for RA. (3) FR elevated: >20 UI/mL. (4) FR negative: ≤20 UI/mL. (5) Anti-CCP elevated: >20 UI/mL. (6) Anti-CCP normal: 0−20 UI/mL. (7) First-line drugs for treatment of FM: pregabalin, duloxetine, tramadol (with or without paracetamol), amitriptyline, cyclobenzaprine. (8) Modified fibromyalgia impact questionnaire (FIQR): test measuring response to treatment in patients with FM. (9) Good response to FM treatment: FIQR <50. (10) Poor response to FM treatment: FIQR >70. Patients with primary FM and elevated RF were carefully examined and underwent laboratory testing, excluding those that had any concomitant autoimmune diseases, mainly RA.
Pearson’s χ2 test for homogeneity was applied to relate those variables indicating improvement with FM treatment and progression to RA in subjects with FM and elevated RF. The ethics committee of our hospital approved the performance for this work.
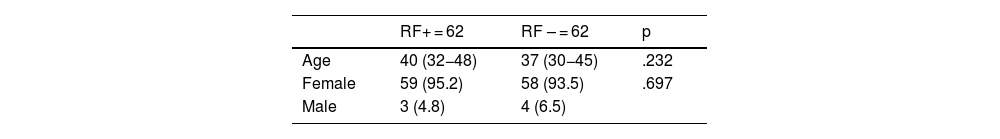
ResultsClinical characteristicsThe results for distribution according to age and sex are displayed in Table 1. The mean age of the cohort was 38.5 years (RF+: 40 years, RF−: 37 years); of the total patient sample of 124 individuals, only 7 were male (ratio: 1/16). No significant relationship was established in terms of age or sex in either group.
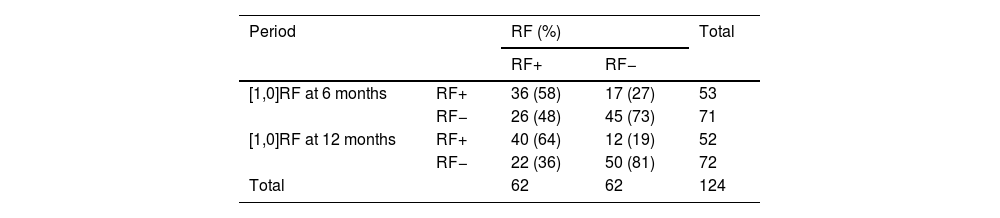
RF behaviour in both groupsChanges in RF at 6 and 12 months were also quantified. RF+ group: of the 62 patients in whom RF was initially elevated, 36 (58%) remained seropositive at 6 months and 40 (64%) at 12 months, with the highest values being 47 and 40 UI/mL at 6 and 12 months, respectively. The RF− group: of the 62 cases who were RF negative at baseline, 17 (27%) became seropositive at 6 months and 12 (19%) at 12 months; the maximum RF levels in this group were 40 and 27 IUI/mL at 6 and 12 months, respectively. Table 2 presents a summary of the data.
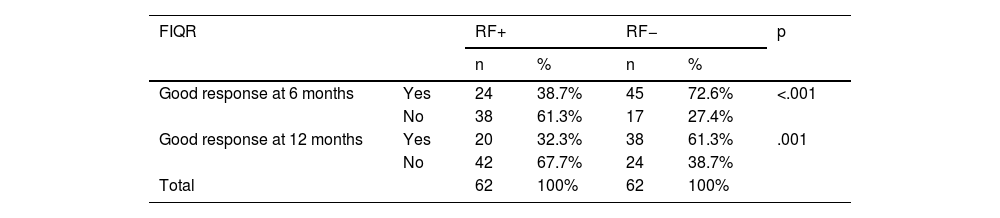
Response to FM therapy in both groupsTable 3 displays the results obtained at this point. All the participants in the study had FIQR scores of >70 at baseline. The FR+ group: of the 62 cases, 24 had an FIQR score of <50 (good response to FM treatment) at 6 months, decreasing to 20 subjects at 12 months. This contrasts with the RF− group; specifically, of the 62 patients, 45 had an FIQR score of <50, which declined to 38 at 12 months and a statistically significant difference at both 6 and 12 months (p < .001).
FIQR at 6 and 12 months in patients with FM.
| FIQR | RF+ | RF− | p | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Good response at 6 months | Yes | 24 | 38.7% | 45 | 72.6% | <.001 |
| No | 38 | 61.3% | 17 | 27.4% | ||
| Good response at 12 months | Yes | 20 | 32.3% | 38 | 61.3% | .001 |
| No | 42 | 67.7% | 24 | 38.7% | ||
| Total | 62 | 100% | 62 | 100% | ||
Pearson’s χ2 for homogeneity; p < 0.05 significant; Good response: FIQR < 50.
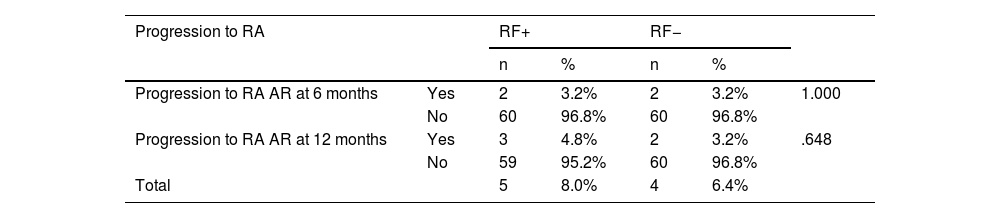
No statistically significant association was identified at this time. In the RF+ group: of 62 individuals, 2 and 3 went on to receive a diagnosis of definite RA at 6 and 12 months, respectively, exhibiting RF values 4 times their normal value (0−20 UI/mL) in all of them. In the RF− group: of the 62 cases, 2 (6 months) and 2 (12 months) progressed to RA. Table 4 sets out the findings at this point.
Progression to RA in subjects with FM at 6 and 12 months.
| Progression to RA | RF+ | RF− | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Progression to RA AR at 6 months | Yes | 2 | 3.2% | 2 | 3.2% | 1.000 |
| No | 60 | 96.8% | 60 | 96.8% | ||
| Progression to RA AR at 12 months | Yes | 3 | 4.8% | 2 | 3.2% | .648 |
| No | 59 | 95.2% | 60 | 96.8% | ||
| Total | 5 | 8.0% | 4 | 6.4% | ||
Pearson’s χ2 for homogeneity; p < .05 significant.
To the best of our knowledge, this is the first research work on individuals with FM and elevated RF to analyse response to FM treatment and progression to RA. This is the first work of its kind; as a result, there are not results that can be compared with outs.
There is no evidence of inflammation in an individual with FM despite the symptoms of soft tissue pain. FM is a disorder of pain regulation and is often classified as a kind of central sensitisation syndrome; as such, it is regarded as a neurosensorial disorder in which the person is incapable of processing pain at the central level.
Primary FM comprises a clinical syndrome the diagnosis of which requires that conditions having similar syndromes be ruled out. Our study was meticulous is selecting patients with FM and elevated RF, excluding the presence of an autoimmune disease, especially RA. The presence of a RF value of more than twice its normal value (0−20 UI/mL), elevated anti-CCP, inflammatory joint disease, the presence or suspicion of synovitis, prolonged morning stiffness, or good response to therapy with corticoids required that the subject be excluded from participating in the study.
There is a variable percentage of cases of FM that that present with high RF figures. Suk et al.9 conducted a study on thyroid autoimmunity and FM. Samples of thyroid hormones, RF, and antinuclear antibodies were collected from 149 patients with FM. Fourteen of these patients presented elevated RF (9.7%). Our study revealed a higher percentage of individuals with FM and elevated RF: in the group of patients with FM and who were initially negative for RF (n = 62), 27% (n = 17) had developed elevated RF at 6 months; this value decreased to 19% (n = 12) at 12 months.
The treatments that are currently available for FM are not effective. Unfortunately, the therapeutic arsenal for FM is of very scant benefit. The situation worsens when other chronic diseases are present. No work has compared the efficacy of FM treatment in the context of increased RF levels. Our study found that the presence of elevated RF hinders clinical improvement (as regards sleep, pain, fatigue, cognitive impairment) despite the use of first-line FM drugs. This situation may possibly be due to defective tolerance and onset of autoimmunity, such that FM together with elevated RF would constitute a preclinical state of an autoimmune disease, and the time to onset of the definitive symptomatology would differ markedly. This autoimmune issue would exacerbate improvement in a patient with FM.10–12 Compounding the above situation is evidence that FM has its own inflammatory cytokine profile. O’Mahony et al.13 carried out a systematic review and meta-analysis (29 and 22 papers, respectively). They found that FM has selectivity for the pro-inflammatory cytokines TNF-alpha, IL-6, and IL-8, in addition to the anti-inflammatory cytokine IL-10. While it is true that RF is not named in this study, it is clear that the pathogenesis of FM would be associated with autoimmunity and would be no more than an autoimmune disease either thwarted or on the road to becoming an established illness, which would account for the poor response of FM to the anti-inflammatory cytokines IL-10, IL-6, and IL-8.
The presence of an elevated level of RF in a subject with FM compels us to rule out autoimmune diseases and a probable progression to RA. Our study has found that the risk of progression to RA in this type of patient at 12 months of follow-up is zero. While it is true that there is no information available with respect to this point, there are studies that have examined how an FM behaves over time. Adams et al.14 performed an observational study of the course and progression of FM in 76 people over a period of 2 years. At the end of their work, 20 of the subjects (26.3%) ceased to have FM. This is in line with other studies, that have concluded that the clinical features of FM fluctuate over time and even remit in some instances, while FM remains in a changing percentage of cases.15
The results of the present work must be confirmed by other future studies, within the context of a growing sensitive population at risk of experiencing chronic pain.
Our study had limitations: it was single-centre, the follow-up time was short (12 months), and there is the possibility of selection bias; to mitigate these limits, all patients who met the selection criteria were included.
ConclusionsIndividuals with FM and elevated RF are more likely to respond worse to treatment, albeit they have a low risk of progressing to RA.
RecommendationsIn a person with FM and elevated RF, the possibility of combining two or more first-line drugs for FM should be evaluated. Although a low likelihood of progression to RA was observed, a rigorous physical examination and regular assessment of RF and anti-CCP is recommended in such cases.
FundingThis study was self-funded.
CRediT authorship contribution statementFLP: conceptualisation, methodology, validation, research lead, resourcing, drafting, writing, visualisation, supervision, project management. JLG: conceptualisation, validation, writing. DSR: methodology, writing. EHB, GOA, ILM, PJR, JYC: drafting, writing, visualisation.
To the relatives of the authors of this work, who bore the brunt of the time spent by their relative on carrying out this research.
This work was an oral presentation at the National Rheumatology Conference in Lima, Peru, in October 2022.