Rheumatoid arthritis (RA) is a chronic autoimmune disease that is characterized by the presence of different autoantibodies such as rheumatoid factor (RF) and anti-citrullinated protein antibodies. CD4 T cells expressing CXCR5, referred as follicular helper T cells (Tfh), collaborate with B cells to produce antibodies. Differential expression of CXCR3 and CCR6 within CD4+CXCR5+ T cells defines three mayor subsets: CXCR3+CCR6− (Tfh1), CXCR3−CCR6− (Tfh2) and CXCR3−CCR6+ (Tfh17). The aim of the study was to assess whether there is an association between the percentage of these cells and RA and whether there is a correlation with disease activity.
Material and methodsTwenty-four RA patients, 22 healthy controls (HC) and 16 undifferentiated arthritis (UA) patients were included. Percentage of CD4+CXCR5+ T cells and their subsets were analyzed by flow cytometry.
ResultsNo differences were found in the percentages of CD4+CXCR5+ T cells in the comparison of RA vs HC or RA vs UA patients. Tfh1, Tfh2 and Tfh17 subsets showed no differences either. There was no correlation between CD4+CXCR5+ T cells, Tfh1, Tfh2 and Tfh17, and Disease Activity Score in 28 joints (DAS28) or erythrocyte sedimentation rate. Surprisingly, there was a positive correlation between Tfh17 cells and C-reactive protein. Finally, there was no correlation between CD4+CXCR5+ T cells, or their subsets, and anti-mutated citrullinated vimentin, or between the cells and RF.
ConclusionThere were no differences between the percentages of CD4+CXCR5+ T cells and their subsets in peripheral blood of RA patients and the percentages of cells in the control groups. This finding does not rule out a pathogenic role of these cells in the development and activity of RA.
La artritis reumatoidea (AR) es una enfermedad autoinmune y crónica caracterizada por la presencia de autoanticuerpos como factor reumatoide (FR) y anticuerpos antiproteínas citrulinadas. Una población de células T helper foliculares (Tfh), que expresan CD4+CXCR5+, colabora con las células B para la producción de anticuerpos. La expresión diferencial de CXCR3 y CCR6 dentro de las células CD4+CXCR5+ define 3 subpoblaciones mayores: CXCR3+CCR6− (Tfh1), CXCR3−CCR6− (Tfh2) y CXCR3−CCR6+ (Tfh17). El objetivo del estudio fue evaluar si existe asociación entre el porcentaje de estas células y la AR, y la correlación de las mismas con actividad de la enfermedad.
Material y métodosParticiparon 24 pacientes con AR, 22 controles saludables (CS) y 16 pacientes con artritis indiferenciada (AI). Los porcentajes de las células CD4+CXCR5+ y sus subpoblaciones fueron analizados por citometría de flujo.
ResultadosNo hubo diferencias en los porcentajes de células CD4+CXCR5+ entre los pacientes con AR y CS o entre AR y AI. Tampoco en las subpoblaciones Tfh1, Tfh2 y Tfh17. No hubo correlación entre las células T CD4+CXCR5+, Tfh1, Tfh2 y Tfh17 y el «Disease Activity Score in twenty-eigth joints» (DAS28), así como tampoco con la velocidad de sedimentación globular. Sorpresivamente, hubo una correlación positiva entre las células Tfh17 y la proteína C reactiva. Finalmente, no hubo correlación entre las células TCD4+CXCR5+ o cualquiera de las subpoblaciones y antivimentina mutada citrulinada así como tampoco entre dichas células y el FR.
ConclusiónNo se hallaron diferencias entre los porcentajes de las células T CD4+CXCR5+ y sus subpoblaciones en sangre periférica de los pacientes con AR y las células de los grupos controles. Esto no descarta un papel patogénico de estas células en el desarrollo y actividad de la AR.
Rheumatoid arthritis (RA) is a chronic, inflammatory, autoimmune disease that does not only affect joints and bones, but is also associated with general complications and with early death.1,2 It is characterized by the presence of circulating autoantibodies, which include rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibodies.2,3 Authors recently described a subpopulation of cluster of differentiation (CD) 4+ T cells present in B-cell follicles, the so-called follicular helper T (Tfh) cells, specialized in collaborating with the B cell for the production of antibodies.4–8 These cells express the chemokine receptor CXCR5, which enables their migration to B-cell follicles in response to its ligand CXCL13.4–9 The distinctive features of Tfh cells include “B-cell lymphoma 6 protein” (Bcl6), “programmed cell death protein 1” (PD-1), “inducible T-cell costimulator” (ICOS) and “CD40 ligand” (CD40L), as well as interleukin 21 (IL-21) release.4–6 However, these cells were defined not only by their phenotypical markers, but also by their anatomical location in the secondary lymphoid organs, which means that their routine analysis in humans is complicated.4,8 A population of circulating helper T cells has been defined that expresses CXCR5 and has a functionality and phenotypical markers similar to those of the Tfh cells that reside in the tissues.10–16 They are heterogeneous and include a number of subpopulations that secrete the cytokines characteristic of T helper (Th) 1, Th2 and Th17, and can be discriminated by CXCR3 and CCR6 serving as surface markers: CXCR3+CCR6− (Th1-like), CXCR3−CCR6− (Th2-like) and CXCR3−CCR6+ (Th17-like).10,16 The increase in Tfh cells has been implicated in the development of systemic autoimmunity in mice.17 In humans, there have been studies of the pathogenic role of these cells in different autoimmune diseases, such as systemic lupus erythematosus,11,12 Sjögren's syndrome,11,18 juvenile dermatomyositis10 and even RA,12,19–23 which found that conflicting results were obtained. The objective of this report was to determine whether CD4+CXCR5+ T cells and their different subpopulations (CXCR3+CCR6−, CXCR3−CCR6−, CXCR3−CCR6+) were associated with RA and whether they were correlated with disease activity, as well as with other prognostic parameters, like autoantibodies and acute-phase reactants.
Materials and MethodsPatients and ControlsWe conducted a cross-sectional case-control study. The patients were admitted by the rheumatology department of Hospital Nacional de Clínicas, Universidad Nacional de Córdoba, Argentina, between March 2014 and February 2015. We included 24 RA patients between the ages of 18 and 70 years, diagnosed according to the 2010 criteria of the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR),24 15 of whom had not been treated, as well as 9 who had been diagnosed more than 12 months earlier and had discontinued their medication for different reasons, and had not received immunosuppressive or immunomodulators for at least 2 months before their visit to the rheumatology clinic. We excluded patients who had taken rituximab within the preceding year or with doses of glucocorticoids greater than 10mg of prednisone/day or its equivalent in another glucocorticoid of a different strength, as well as those who had received a viral or bacterial vaccine within the preceding 2 months, those with other associated autoimmune diseases, acute processes or neoplasms, and pregnant women. At the time of admission, the disease activity was evaluated using the Disease Activity Score in 28 joints (DAS28) in terms of 4 aspects: tender and swollen joint counts, visual analogue scale and erythrocyte sedimentation rate (ESR).25 As control groups, we included a series of 16 patients with undifferentiated arthritis (UA) who had clinical arthritis but did not meet the criteria for a diagnosis of RA or other inflammatory arthropathies at the time they were enrolled in this study—and with whom we took into account the same selection criteria as for RA patients—and a group of healthy controls (HC) composed of 22 voluntary adults, within the same age range and having the same sex distribution as the patients; these individuals had not been diagnosed with autoimmune diseases, acute viral or bacterial processes, endocrine diseases or neoplasms, and had not received antibiotics or viral or bacterial vaccines within the preceding 2 months. All of the participants in the study gave their written consent. This proposal was approved by the institutional research ethics committee of Hospital Nacional de Clínicas, Universidad Nacional de Córdoba, Argentina.
Laboratory TestsWhole blood samples anticoagulated with EDTA-K2 were utilized for cytological analyses and serum samples were employed for the remainder of the laboratory tests. Complete cytological analyses were performed utilizing the Counter 19/19CP automated analyzer (Wiener Laboratories, Sociedad Argentina de Investigación Clínica [SAIC], Rosario, Argentina) and differential counts with May–Grünwald–Giemsa stain. Rheumatoid factor was detected using, Artritest, a latex agglutination assay (Wiener Laboratories [SAIC], Rosario, Argentina), employing slices=1/20. The determination of C-reactive protein (CRP) was done with a particle-enhanced turbidimetric immunoassay (Siemens, New York, New York, United States), employing the Siemens Dimension RxL Max autoanalyzer (New York, New York, United States); reference value (RV): ≤9mg/L. Anti-mutated citrullinated vimentin (anti-MCV) antibodies were detected using an enzyme-linked immunosorbent assay (Orgentec Diagnostika GmbH, Mainz, Germany). The procedure was carried out according to the manufacturer's instructions. A positive result was a value ≥20U/mL. Finally, immunoglobulins (RV for immunoglobulin [Ig] G, 650–1370mg%; for IgM, 25–198mg%; and for IgA, 35–350mg%) and complement components C3 (RV 80–180mg%) and C4 (RV 17–40mg%) were measured by means of single radial immunodiffusion.
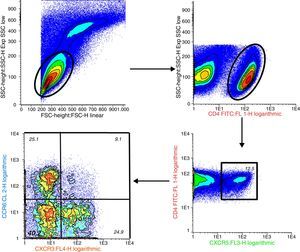
Determination of CD4+CXCR5+ T Lymphocytes and Their Distinct Subpopulations by Flow CytometryPeripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation using Ficoll-Paque™ Plus (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). For immunostaining, we utilized 1.16 PBMC and the following antibodies: anti-human CD4 fluorescein isothiocyanate (FITC) (clone RPA-T4), anti-human CD196 (CCR6) phycoerythrin (PE) (clone 11A9), anti-human CD183 (CXCR3) allophycocyanin (APC) (clone IC6/CXCR3) and anti-human CXCR5 peridinin chlorophyll protein (PerCP-Cy™5.5) (clone RF8B2) (BD Pharmingen™, San José, California, United States). The data were acquired immediately after staining with a FACSCalibur 4-color flow cytometer (Becton-Dickinson, Boston, Massachusetts, United States). In each sample, we analyzed at least 50,000 CD4+ cells using the Infinicyt™ software package (version 1.7) (Citognos SL, Salamanca, Spain).
Statistical AnalysisThe data were analyzed with the MedCalc software package (version 10.2.0.0) (MedCalc Software, Ostend, Belgium). The normal distribution of the data was assessed with the Kolmogorov–Smirnov test. For the variables with a normal distribution or whose logarithmic transformation had a normal distribution, we used parametric tests like one-way analysis of variance (ANOVA) and the Bonferroni correction, as well as the Pearson correlation coefficient. For the remaining variables, we employed nonparametric methods, such as the Kruskal–Wallis and Dunn tests, and the Spearman correlation coefficient. The categorical data were compared with the Chi-square test. A P value <.05 was considered to indicate statistical significance.
ResultsCharacteristics of the study groups:Table 1 shows the demographic and clinical characteristics of the study groups. The ages and sex distribution were homogeneous. As was expected, the ESR and CRP levels were higher in the patients with RA when compared to the HC. In all, 79% of the RA patients were positive for RF, whereas none of the controls had a positive test. Likewise, the anti-MCV autoantibody levels were significantly higher in RA patients when compared to the other 2 groups. The IgM levels were higher in patients with RA when compared to those of the group of HC, and those of IgA in the comparison with patients with UA. The levels of complement C4 were lower in RA patients when compared to those with UA, but remained in normal range in both groups. Most of the patients with RA had high disease activity (Table 2). Two patients, 1 of whom was in remission, had a low activity (DAS28<2.6).26
Summary of the Characteristics of the Controls and Patients Participating in the Study.
| RA (n=24) | HC (n=22) | UA (n=16) | RA vs HC, P value | RA vs UA, P value | |
|---|---|---|---|---|---|
| Sex, F/M | 21/3 | 19/3 | 13/3 | .77c | .93c |
| Age, years | 51±10 | 49±10 | 52±11 | >.05d | >.05d |
| WBC,a 109/L | 6.98±1.85 | 7.23±1.96 | 6.84±1.74 | >.05d | >.05d |
| Hb,a g/L | 12.6±1.91 | 12.6±0.98 | 12.96±1.07 | >.05e | >.05e |
| PLT,a 109/L | 255±80 | 254±41 | 249±59 | >.05e | >.05e |
| ESR,a mm/h | 25±21 | 9±6 | 14±12 | <.01e | >.05e |
| CRP,a mg/L | 18±14 | 9±2 | 14±8 | <.01e | >.05e |
| RF+, n (%) | 19 (79) | 0 (0) | 0 (0) | <.0001c | <.0001c |
| MCV,b U/mL | 75.0 (7.7–530.0) | 2.8 (2.3–5.3) | 2.9 (2.5–3.8) | <.001e | <.001e |
| IgG,a mg% | 1310±338 | 1325±257 | 1212±336 | >.05c | >.05c |
| IgM,a mg% | 220±88 | 169±55 | 179±60 | <.05c | >.05c |
| IgA,a mg% | 363±126 | 313±80 | 261±114 | >.05c | <.05c |
| C3,a mg% | 112±33 | 119±26 | 130±30 | >.05c | >.05c |
| C4,a mg% | 24±9 | 26±7 | 31±8 | >.05c | <.05c |
| DAS28a | 5.16±1.35 | – | – | – | – |
Anti-MCV, anti-mutated citrullinated vimentin antibodies; CRP, C-reactive protein; DAS28, Disease Activity Score in 28 joints; ESR, erythrocytes sedimentation rate; F, female; Hb, hemoglobin; HC, healthy controls; Ig, immunoglobulin; M, male; PLT, platelets; RA, rheumatoid arthritis; RF, rheumatoid factor; UA, undifferentiated arthritis; WBC, white blood cells.
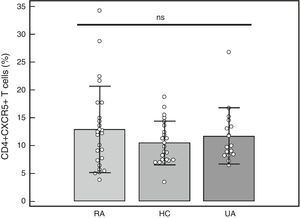
Percentage of CD4+CXCR5+T cells: To identify the distinct subpopulations of these cells by flow cytometry, we applied the strategy detailed in Fig. 1. We compared the percentage of CD4+CXCR5+ T cells in the 3 groups, and found no significant differences (RA: 12.89%±7.73%, HC: 10.48%±3.90%, UA: 11.71%±5.04%; P=.66), as shown in Fig. 2. Likewise, there were no differences between the groups when we considered only the patients with high activity (DAS28>5.1, n=14; 12.84%±8%; P=.73).
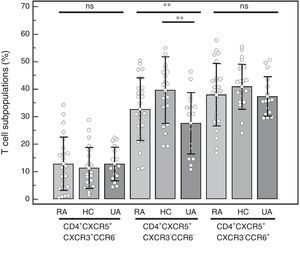
CD4+CXCR5+T cell subpopulations: We compared the percentages of the distinct subpopulations of CXCR3+CCR6− (Tfh1), CXCR3−CCR6− (Tfh2) and CXCR3−CCR6+ (Tfh17) cells within the CD4+CXCR5+ population (Fig. 3). We observed no differences in the Tfh1 subpopulation (RA=12.75%±9.72%, HC=11.21%±7.48%, UA=12.81%±6.13%; P=.77), or in the Tfh17 subpopulation (RA=37.94%±11.34%, HC=40.79%±8.17%, UA=37.34%±7.16%; P=.45). The difference observed in the Tfh2 subpopulation (RA=32.66%±11.46%, HC=39.53%±12.12%, UA=27.56%±11.25%; P=.0092) was due to the lower percentage found in the UA group when it was compared to the HC (P<.01). No differences were observed when it was compared only with those patients with RA who had high disease activity (n=14) and the control groups (Tfh1 in RA was 12.74%±9.19%, P=.77; Tfh2 in RA was 35.03%±11.64%, P<.01; Tfh17 in RA was 36.21%±10.80%, P=.26), again, and as was expected, the difference in Tfh2 was due to the results on comparing UA vs HC (P<.01).
Percentage of the distinct CD4+CXCR5+ T cell subpopulations in peripheral blood mononuclear cells from patients with rheumatoid arthritis (RA), healthy controls (HC) and patients with undifferentiated arthritis (UA). One-way analysis of variance and the Bonferroni post-test. ns: not significant. **P<.01.
Correlation of the study populations and disease activity: We analyzed the correlation between the CD4+CXCR5+ population and its distinct subpopulations with the DAS28. We found no correlation between the CD4+CXCR5+ cells and the DAS28 (r=−0.19, P=.37), or between the Tfh1, Tfh2 and Tfh17 subpopulations and the activity index employed (Tfh1 and DAS28 r=0.09, P=.68; Tfh2 and DAS28 r=−0.36, P=.09; Tfh17 and DAS28 r=−0.20, P=.35). When we took into account only those patients with high disease activity (n=14), we observed no correlation between any of the subpopulations being studied and the DAS28 (CD4+CXCR5+ and DAS28 r=−0.32, P=.26; Tfh1 and DAS28 r=−0.17, P=.56; Tfh2 and DAS28 r=0.33, P=.25; Tfh17 and DAS28 r=0.024, P=.94).
Correlation between Tfh cells and the different autoantibodies in patients with RA: Given that Tfh cells collaborate with B cells for the production of antibodies, we proposed to determine whether CD4+CXCR5+ T cells and their distinct subpopulations could be related to the production of autoantibodies in RA patients. We found no correlation between the percentages of CD4+CXCR5+ T cells (r=0.38, P=.066), or between the 3 subpopulations (Tfh1 r=−0.04, P=.84; Tfh2 r=−0.14, P=.51; Tfh17 r=−0.19, P=.37) and anti-MCV antibodies. The same result was observed when the percentages of these cell populations were correlated with RF (CD4+CXCR5+: r=0.30, P=.15; Tfh1 r=−0.18, P=.38; Tfh2 r=−0.15, P=.46; Tfh17 r=0.0051, P=.98).
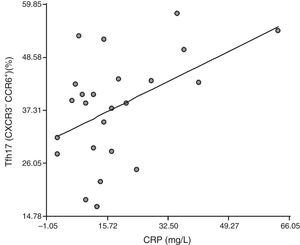
Correlation between Tfh cells and the distinct cell subpopulations with inflammation markers: To establish whether Tfh cells and the different subpopulations had a role in the inflammatory process that occurs in RA patients, we correlated them with ESR and CRP. We detected no correlation between the CD4+CXCR5+ population and ESR or CRP (r=0.18, P=.39 and r=0.27, P=.20, respectively). We found no correlation between Tfh1, Tfh2 and Tfh17 and ESR (r=0.080, P=.71; r=−0.0094, P=.97 and r=−0.25, P=.23, respectively), or between Tfh1 and CRP (r=0.27, P=.20) or Tfh2 and CRP (r=0.14, P=.51). Surprisingly, there was a positive correlation between Tfh17 and CRP (r=0.47, P=.021) (Fig. 4).
DiscussionAlthough a number of immunopathogenic mechanisms have been proposed, the cause of RA is still unknown. As a consequence, the discovery of both the elements of the immune system that participate in its onset and the mechanisms involved is of great value for the development of more effective therapies. In our investigation, we studied a subpopulation of CD4+ T cells, Tfh cells, which have a very important role in the collaboration for the production of antibodies by the B cells. However, we found no significant differences in the percentage of CD4+CXCR5+ T cells in the blood of RA patients when compared with that of the HC or of patients with UA. The same can be said when we considered only patients with RA and high disease activity. The literature provides relatively variable results that may be associated with the manner of characterizing this population in peripheral blood. Our findings agree with those reported by Chakera et al., who found no differences when they compared Tfh cells such as CD4+CXCR5+, CD4+CD45RO+CXCR5+, CD4+CXCR5+ICOShigh and CD4+CXCR5+PD-1high.20 The authors of another study observed similar percentages of CD4+CXCR5+CD45RA− and CD4+CXCR5+CD45RA−CCR7loPD-1high cells.12 Likewise, according to an article by Arroyo-Villa et al., no differences were found when CD4 and CXCR5 were employed to characterize them.21 However, when ICOS was added to the original labeling, a greater number of CD4+CXCR5+ICOS+ cells was observed in RA patients.21 Similarly, in contrast to our findings, the literature includes other studies that report an increase in the number of Tfh cells in RA patients after the addition of ICOS as a third cell marker, as occurred even with PD-1 when associated with the abovementioned markers.19,22,23 Previous studies of CD4+CXCR5+ T cells from mouse germinal centers identified a population that expresses the master regulators Bcl6 and Foxp3, with the capacity to prevent antibody-mediated autoimmunity, referred to as follicular regulatory T cells (TFR).27 Recently, this population was also reported in human blood and tonsils.28,29 As the Tfh and TFR cells fulfill opposing functions and share the expression of a number of markers, such as CXCR5, ICOS and PD-1, the addition of the use of Foxp3 as a marker would be crucial to be able to discriminate them. This would help to reconcile apparently contradictory findings in relation to the consequences of numerical changes in these populations observed in the course of autoimmune processes.
To define the Tfh cell subpopulations, we employed the combination of markers CCR6 and CXCR3, in accordance with the criteria employed by Morita et al. in 2011.10 That group of researchers and another team found that subpopulations Tfh2 and Tfh17 were effective collaborators of B cells for the induction of the synthesis of distinct immunoglobulin isotypes.10,21 As RA is a disease involving autoantibody production, we expected to observe an increase in Tfh2 and Tfh17 subpopulations; however, we detected no variation in either of the two, or in the Tfh1 subpopulation. In this respect, the literature also reports contradictory results in the study of patients with RA. The group of Arroyo-Villa et al. found a difference in the number of Tfh17 cells, but not in that of Tfh2,21 whereas another group reported no changes among any of the subpopulations in RA patients.20
In the study of the comparisons of the DAS28, CRP, ESR, RF and anti-MCV antibodies in the distinct populations, we detected no correlation in any case, except between Tfh17 and CRP. Similar to what occurred in this study, in the literature there are no previous reports of a correlation between Tfh cells and parameters of disease activity like the DAS28, CRP and ESR.23 In contrast, other groups observed a positive correlation between Tfh and the DAS2822,23 or between Tfh and anti-cyclic citrullinated peptide antibodies19,22 and Tfh and RF.19 With regard to the correlation between Tfh17 and CRP, an earlier article demonstrated that the Tfh17 subpopulation produces IL-21, IL-17A and IL-22.10 These cytokines could indirectly promote the production of CRP as they stimulate hepatocytes, keratinocytes and epithelial cells to produce IL-1β and IL-6,30 that act in the liver to induce CRP synthesis,2 which could be the mechanism employed in RA.
In conclusion, we found no numerical differences between Tfh cells and the distinct subpopulations in peripheral blood or any correlation with parameters of disease activity and inflammatory markers. Nevertheless, we cannot rule out the possibility that they play a pathogenic role in RA. This population could be recruited to the site of inflammation, the joint in this case, and collaborate with B cells for the production of autoantibodies in ectopic germinal centers, located in the synovial membrane of RA patients.
A clear phenotypic description of memory Tfh cell subgroups in the blood is important, not only to help understand their biological functions, but also for translational purposes, as these circulating cells could serve as potential biomarkers to monitor the deregulated responses of antibodies in autoimmune diseases like RA.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThe funds required to perform the work for this report were provided by the Sociedad Argentina de Reumatología.
Conflicts of InterestThe authors declare they have no conflicts of interest.
We wish to thank biochemists Cecilia María Rodriguez and Melina Cloquell for collaborating in the analysis of the flow cytometry samples.
Please cite this article as: Costantino AB, del Valle Acosta C, Onetti L, Mussano E, Cadile II, Ferrero PV. Células T helper foliculares en sangre periférica de pacientes con artritis reumatoidea. Reumatol Clin. 2017;13:338–343.