Antibodies against neutrophil cytoplasm (ANCA) are associated with vasculitis. There are different methods to determine their presence. The interference of antinuclear antibodies (ANA) in the differentiation between P-ANCA and C-ANCA patterns has been described.
ObjectiveTo determine the frequency of ANCA in a population with manifestations of autoimmune disease, and evaluate the interference of ANA in its interpretation.
Materials and methodsRetrospective, descriptive nonexperimental cross-sectional study, including 3330 data. The presumptive diagnosis was autoimmune disease and a test for ANCA was requested. The ANCA and ANA determinations were made by indirect immunofluorescence, L-ANCA® and CytoBead® ANCA. Anti-proteinase 3 and anti-myeloperoxidase were detected by ELISA and CytoBead® ANCA.
ResultsANCAs were positive in 10.21% and 12.64% of those positive for ANCA were positive for ANA. The inter-rater agreement statistic (Kappa) for anti-PR3 between CytoBead ANCA and ELISA was 100% (K=1.00; P<.05) and the agreement between anti-myeloperoxidase by ELISA and CytoBead® ANCA was high (K=0.94; P<.05). 30% of those with ANCAs had a diagnosis of a type of vasculitis; 20% of them had an autoimmune disease.
ConclusionsThe results suggest an overestimated request for ANCAs as a diagnostic aid in primary care which was not addressed. For an adequate evaluation of ANCAs, the indirect immunofluorescence technique should be implemented for the control and confirmation with the determination of specific antigens for anti-proteinase 3 and anti-myeloperoxidase in any of the confirmatory assays. The high concordance shown by ANCA CytoBeads makes us consider the use of this alternative for the determination of ANCAs and the confirmation. Given the interference of ANAs, the ANA test by IFI in the presence of positive P-ANCA results is recommended in order to minimise “false positives”.
Los anticuerpos anticitoplasma del neutrófilo (ANCA) se asocian con vasculitis. Existen diferentes métodos para determinar su presencia. Se ha descrito la interferencia de anticuerpos antinucleares (ANA) en la diferenciación de los patrones P-ANCA y C-ANCA.
ObjetivoDeterminar la frecuencia de ANCA en una población con manifestaciones de enfermedad autoinmune; y evaluar la interferencia de los ANA en su interpretación.
Materiales y métodosEstudio de corte transversal retrospectivo, descriptivo no experimental incluyendo 3.330 datos con diagnóstico presuntivo de enfermedad autoinmune y solicitud de ANCA. Las determinaciones de ANCA y de ANA se realizaron mediante inmunofluorescencia indirecta, L-ANCA® y CytoBead® ANCA. Antiproteinasa 3 y antimieloperoxidasa fueron determinados mediante ELISA y CytoBead® ANCA.
ResultadosSe encontraron ANCA positivos en el 10,21% y el 12,64% con ANCA positivos presentaban ANA positivos. La concordancia kappa para antiproteinasa 3 entre CytoBead® ANCA y ELISA fue del 100% (K=1; p<0,05), La concordancia entre antimieloperoxidasa por ELISA y CytoBead® ANCA fue alta (K=0,94; p<0,05). El 30% de aquellos con ANCA positivos tenía diagnóstico de algún tipo de vasculitis, el 20% cursaba con alguna enfermedad autoinmune.
ConclusionesLos resultados indican una solicitud sobreestimada de este marcador como ayuda diagnóstica en consulta de atención primaria no direccionada. Para una adecuada evaluación de ANCA se debe implementar la técnica de inmunofluorescencia indirecta para tamizaje y confirmar con la determinación de antígenos específicos para antiproteinasa 3 y antimieloperoxidasa por cualquiera de los ensayos confirmatorios. La alta concordancia mostrada por CytoBeads® ANCA hace que planteemos el empleo de dicha alternativa para la determinación de ANCA y su confirmación. Dada la interferencia de los ANA, se recomienda solicitar la prueba ANA por inmunofluorescencia indirecta ante la presencia de resultados P-ANCA positivos, con el fin de minimizar «falsos positivos».
Anti-neutrophil cytoplasmic antibodies (ANCA) target primary neutrophil and monocyte granules.1 Towards 1985, ANCA were associated with vasculitis such as granulomatosis with polyangiitis (GPA), formerly called Wegener's granulomatosis, among others including a large number of inflammatory and infectious diseases.2–4 ANCA testing has become an established diagnostic aid for evaluating small vessel necrotising vasculitis.5
There are traditionally 2 methods for determining the presence of ANCA.6 The most commonly used method is the indirect immunofluorescence assay (IIF), defining two main patterns: C-ANCA, which has a cytoplasmic pattern and most target proteinase 3 (PR3) and P-ANCA, which has a perinuclear pattern and target myeloperoxidase (MPO).7 However, this methodology has drawbacks when differentiating between P-ANCA and C-ANCA patterns when it is next to antinuclear antibodies (ANA). Due to this interference, ANA determination in conjunction with ANCA8 and the presence of ANCA targeting antigens other than MPO or PR3 are important.
The second method for specific testing is the enzyme-linked immunosorbent assay (ELISA), which identifies antibodies that target specific antigens such as PR3 and MPO.9,10 The sensitivity of the IIF is between 80% and 90% while its specificity is less than 80% due to the presence of P-ANCA targeting antigens other than MPO.6 For this reason, the ANCA consensus suggests using the IIF technique as screening and then performing a confirmatory test against specific antigens by means of ELISA.11
Another test has recently emerged that uses IIF to test for ANCA called CytoBead® ANCA; this test contains human granulocytes fixed in ethanol and also integrates microparticles coated with PR3 antigen and MPO as substrate, generating their specific identification and has become a good alternative to consider in identifying ANCA since it has optimal sensitivity and specificity in one test.12–14
For this reason, the main objective of this study was to determine the frequency of positive ANCA in a population with manifestations of autoimmune disease; and to evaluate the interference of ANA in their interpretation.
Materials and methodsA retrospective, descriptive, non-experimental cross-sectional study. We have included 3330 pieces of data collected from patients with a presumptive or suspected diagnosis of autoimmune disease and request for ANCA between 2013 and 2015, referred to the Hospital Militar Central and the Instituto de Referencia Andino. In addition, to compare the performance of 3 methods, 44 samples were selected with a previous ANCA request where the presence of ANCA and ANA was determined simultaneously by means of different methodologies using the L-ANCA®, CytoBead® ANCA kits, and anti-PR3 and anti-MPO by ELISA. The project was approved by the Research and Ethics Committee of the Hospital Militar Central (code N 2014-080).
Statistical methodsDemographic characteristics, age, gender and clinical manifestations were analysed by frequency using SPSS V18 for Windows, with a 95% confidence level, and the STATA 11® programme, agreement between the techniques was tested by the Kappa coefficient. The frequency of signs and symptoms and the clinical areas referring the request were established. ANCA positivity was then distributed according to the clinical manifestations of autoimmune disease.
L-ANCA® (Ref. 10070-L-11, Immunoconcepts®)All the serum samples were adjusted to a 1:20 dilution. The L-ANCA® test contains granulocytes and a lymphocyte content, which when viewed under the microscope will indicate the presence of positive ANA in the samples simultaneously with ANCA – if positive.15 Only the presence of ANA was reported without identifying the pattern in the case of positivity, given the cell substrate used.
CytoBead® ANCA (Ref. 8063.GA Generic Assays)The CytoBead® ANCA kit is a multiplex assay containing human granulocytes fixed in ethanol as well as microparticles coated with PR3 antigen and MPO as substrate which enable the identification of positive ANCA differentiating C-ANCA from P-ANCA specifically.16
ELISA (®Anti PR3 y anti MPO, Ref 4058,4059 Generic Assay GmbH)IgG antibodies against PR3 and MPO were identified in human serum with positive values above 10IU/ml. Anti-PR3 and anti MPO-ANCA were detected using commercially available antigen-specific ELISAs according to the manufacturer's instructions.
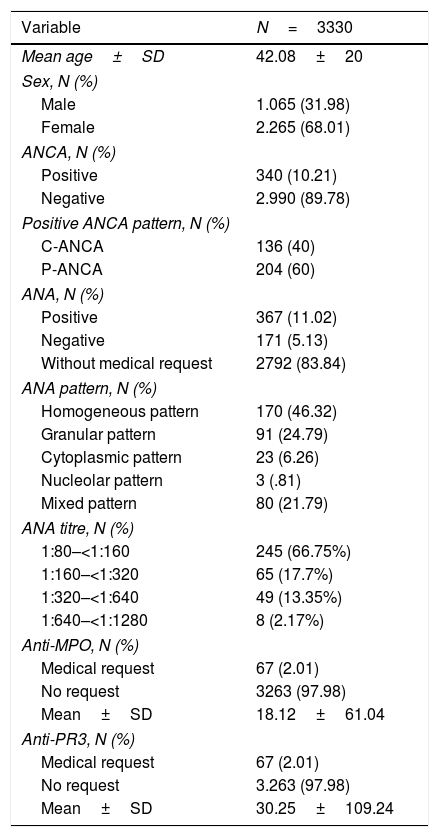
ResultsDescription of the sampleWe retrospectively analysed 3330 data from patients with an ANCA request. Of the total requests, 1065 (31.9%) corresponded to male patients and 2265 (68%) to female patients, with an average age of 42.08±20. ANCA positivity was evidenced in 10.2% (340/3330) of the requests, of which 40% (136/340) had C-ANCA pattern and 60% (204/340) P-ANCA (Table 1).
Demographic and serological variables.
| Variable | N=3330 |
|---|---|
| Mean age±SD | 42.08±20 |
| Sex, N (%) | |
| Male | 1.065 (31.98) |
| Female | 2.265 (68.01) |
| ANCA, N (%) | |
| Positive | 340 (10.21) |
| Negative | 2.990 (89.78) |
| Positive ANCA pattern, N (%) | |
| C-ANCA | 136 (40) |
| P-ANCA | 204 (60) |
| ANA, N (%) | |
| Positive | 367 (11.02) |
| Negative | 171 (5.13) |
| Without medical request | 2792 (83.84) |
| ANA pattern, N (%) | |
| Homogeneous pattern | 170 (46.32) |
| Granular pattern | 91 (24.79) |
| Cytoplasmic pattern | 23 (6.26) |
| Nucleolar pattern | 3 (.81) |
| Mixed pattern | 80 (21.79) |
| ANA titre, N (%) | |
| 1:80–<1:160 | 245 (66.75%) |
| 1:160–<1:320 | 65 (17.7%) |
| 1:320–<1:640 | 49 (13.35%) |
| 1:640–<1:1280 | 8 (2.17%) |
| Anti-MPO, N (%) | |
| Medical request | 67 (2.01) |
| No request | 3263 (97.98) |
| Mean±SD | 18.12±61.04 |
| Anti-PR3, N (%) | |
| Medical request | 67 (2.01) |
| No request | 3.263 (97.98) |
| Mean±SD | 30.25±109.24 |
Together with the initial ANCA requests, only 67 of the 3330 had a request for anti-MPO and anti-PR3, of which 62.7% (42/67) had positive ANCA and 11.9% (8/67) were only positive for anti-PR3, 16.4% (11/67) only for anti-MPO and 5.9% (4/67) had mixed positivity.
ANA were requested with ANCA simultaneously in 538 data, of which 68.2% (367) were positive for ANA only, and a homogeneous pattern was identified in 68% (170), granular in 24.7% (91), cytoplasmic in 6.2% (23), nucleolar in .8% (3) and 21.8% (80) of the results had more than one pattern for ANA, which was termed “mixed pattern”. For the positive data, the titre of these antibodies was tested and the most frequent was found to be between 1:80 and 1:160.
It was found that 12.6% of the data collected retrospectively with positive ANCA also had positive ANA; of these 83.7% corresponded to the P-ANCA pattern and 16.27% to the C-ANCA pattern. In the positive P-ANCA the predominant ANA pattern was the homogeneous pattern followed by the granular pattern, in the C-ANCA the predominant ANA pattern was cytoplasmic followed by granular.
Agreement between techniquesIn order to compare the performance of the methods, 44 samples were selected with a previous ANCA request where the presence of ANCA and ANA was determined by means of different methodologies using the abovementioned L-ANCA®, CytoBead® ANCA kits and anti-PR3 and anti-MPO by ELISA. This group had a distribution of 77.3% females and 22.8% males, with a mean age of 48.27±21.27. Seventy-five percent (33/44) had positive results for previous ANCA, which corresponded to C-ANCA in 36.4% and P-ANCA in 63.6%. In addition, 61.3% (27/44) positivity for ANA was observed.
Thirty-six point four percent (16/44) were positive for ANCA and ANA simultaneously, of which 87.5% (14/16) had a P-ANCA pattern and 12.5% (2/16) C-ANCA. Of the P-ANCA samples only 7 were positive for anti-MPO and 2 for anti-PR3 and the remaining 5 were negative for anti-MPO and anti-PR3.
When the L-ANCA® technique was used, positivity for ANCA was observed in 59.1% (26/44), of which 85.8% (23/26) corresponded to the P-ANCA pattern and 11.5% (3/26) to the C-ANCA pattern; 43.2% (19/44) were positive for ANA using the L-ANCA® methodology and 56.8% (25/44) negative. Sixty-one point five percent 61.5% (16/26) were positive for ANCA and ANA at the same time, of which 93.7% (15/16) had a P-ANCA pattern.
By CytoBead® ANCA, 38.6% (17/44) of the samples were positive for ANCA, and 61.4% (27/44) were negative. In turn, 35.3% (6/17) of the ANCA-positive samples were positive for anti-PR3, 41.2% (7/17) for anti-MPO and 23.5% (4/17) had mixed positivity.
Finally, in the 44 samples with previous ANCA results the concentration of anti-PR3 and anti-MPO was quantified by means of ELISA, obtaining an average of 43.8U/ml for anti-PR3 and 24.7U/ml for anti-MPO. Eighteen positive samples were identified, of which 13.6% (6/44) were anti-PR3 positive, 18.2% (8/44) were MPO positive and 9.1% (4/44) had mixed positivity, with a cut-off point of 10U/ml.
The performance of the difference methods was assessed through a correlation analysis and obtaining the Kappa index for all the cases where ANCA were measured by least 2 of the techniques evaluated.
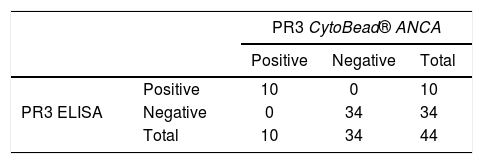
Agreement in the measurement of anti-PR3 between the CytoBead® ANCA technique and ELISA was 100% (K=1, P<.05), of which 10 results were positive and 34 negative for both techniques, without differing results (Table 2).
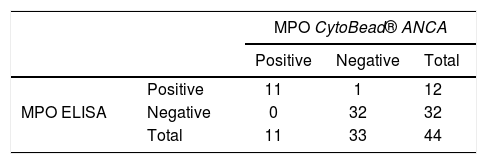
When comparing anti-MPO measurement by ELISA against CytoBead ANCA, discrepancy was observed in one result that was negative by CytoBead ANCA and positive by ELISA with a value of 10.1 taking as the benchmark a cut-off point of 10U/ml, nevertheless agreement was high (K=.94, P<.05) (Table 3).
A discrepancy was observed in the evaluation between CytoBead® ANCA and L-ANCA® in two aspects: first, 14 data that were positive for L-ANCA® were negative for CytoBead® ANCA; second, 5 data negative for L-ANCA® were positive for CytoBead® ANCA, thus obtaining very low agreement (K=.17; P>.05).
When comparing the results obtained by CytoBead® ANCA with previous ANCA results (taken retrospectively) it was observed that of 27 results that were negative by CytoBead® ANCA, 17 of these had previous positive ANCA and 6 of these had previous positive ANA at the same time: ANA were not technically performed previously in the remaining 11 (P=.02). Likewise, of the 18 negative results by L-ANCA®, 16 had previous positive ANCA and 7 of these showed previous positive ANA simultaneously (P=.07). On examining the results obtained by CytoBead® ANCA, L-ANCA® and previous ANCA, only 11 results were observed as positive for the 3 tests.
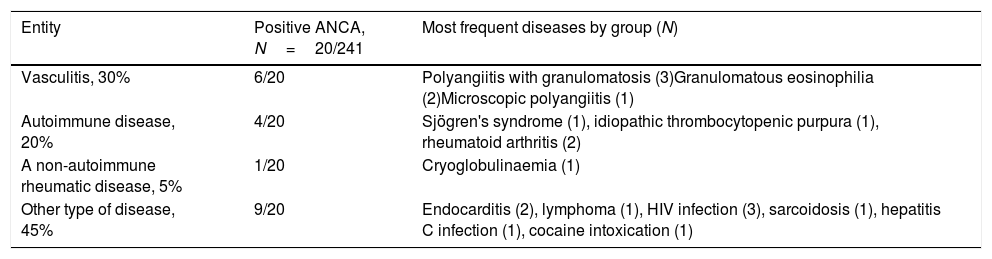
Finally, the clinical histories of 241 out of the 3330 requests with the most frequent clinical manifestations were reviewed for which ANCA were requested and were categorised in 4 groups: the first had some type of vasculitis, the second an autoimmune disease, the third a non-autoimmune rheumatic disease and the fourth another type of disease (Table 4).
Distribution of diseases with positive ANCA by IIF.
| Entity | Positive ANCA, N=20/241 | Most frequent diseases by group (N) |
|---|---|---|
| Vasculitis, 30% | 6/20 | Polyangiitis with granulomatosis (3)Granulomatous eosinophilia (2)Microscopic polyangiitis (1) |
| Autoimmune disease, 20% | 4/20 | Sjögren's syndrome (1), idiopathic thrombocytopenic purpura (1), rheumatoid arthritis (2) |
| A non-autoimmune rheumatic disease, 5% | 1/20 | Cryoglobulinaemia (1) |
| Other type of disease, 45% | 9/20 | Endocarditis (2), lymphoma (1), HIV infection (3), sarcoidosis (1), hepatitis C infection (1), cocaine intoxication (1) |
ANCA positivity was found in 8.3% (20/241), for which the P-ANCA pattern was identified in 90% (18/20) and C-ANCA in 10% (2/20). Of the total requests with a clinical history, positive ANA were found in 35% (85/241) with a homogeneous pattern in 54.1%, granular in 34.1%, cytoplasmic in 10.6% and nucleolar in 1.18%. In the data that were positive for ANCA the simultaneous presence of ANA was identified in 25% (5/20), with a speckled pattern in 40%, homogeneous in 40% and cytoplasmic in 20%.
On retrospectively making the clinical correlation of the patients with positive ANCA it was found that 30% (6/20) had been diagnosed with vasculitis of some type, 20% (4/20) had an autoimmune disease, 5% (1/20) had non-autoimmune rheumatic disease and 45% (9/20) had another type of disease in the primary assessment according to their clinical history.
DiscussionThe annual incidence worldwide of vasculitis is approximately 10–20 cases per million inhabitants with a mortality rate reaching 80%.17,18 GPA-type vasculitis associated with the presence of ANCA does not have homogeneous geographic distribution and the reported prevalence of ANCA in GPA varies between 50% and 95%.14 In the Japanese and Afro-American population the incidence of GPA is lower but there is higher frequency of MPA,19,20 by contrast, GPA is more frequent in northern Europe with fewer documented cases of MPA.17,18 In Colombia, cases of other subtypes of vasculitis and GPA have been identified.19 It is believed that the low percentage of cases reported with a diagnosis of GPA might be related to the poor referral of these patients to rheumatology departments, and treating physicians and the Ministry of Social Protection failing to record these cases.21
In this study, the results showed positive ANCA in 340 data obtained, of which 136 had a C-ANCA and 204 P-ANCA pattern; however, ANA and antibodies targeting other cytoplasmic granules as antigens (lactoferrin, lysozyme, azurocidin, elastase, cathepsin G, bactericidal growth enzyme/permeability) show atypical ANCA patterns, and these have presented some technical difficulty in being differentiated and can be confused with the P-ANCA pattern.9,22,23 In our data, 12.6% of patients with ANA simultaneously presented positive ANCA with a homogeneous pattern at low titres in the majority. Despite being one of the most frequent patterns in autoimmune diseases, this group of patients did not have a diagnosis of systemic lupus erythematosus.24–26
The results taken retrospectively in our study showed predominance of the P-ANCA pattern in the data that were positive for ANA and ANCA, which has been described in the literature as possible false positives for ANCA by IIF due to interference in the process of binding to the substrate that occurs due to a rearrangement of proteins of the nucleus when the samples are fixed in ethanol, generating diffuse colouration; another interference has been described in results with positive anti-DNA antibodies, which simultaneously evidences a need for experts in the correct reading and interpretation of the ANA and ANCA pattern.12,15
The study published by Martinez Tellez et al., sought to determine the positivity and clinical correlation of ANCA, taking into account the interference of ANA; it was found that the likelihood of finding ANCA is greater in patients with positive ANA.8 The atypical P-ANCA pattern is found in patients with inflammatory bowel disease, predominantly ulcerative colitis.23 In combination with anti-Saccharomyces cerevisiae antibodies, P-ANCA are helpful in the differentiation of ulcerative colitis and Crohn's disease, the anti-S. cerevisiae antibody being more typical of Crohn's disease and P-ANCA of ulcerative colitis23; however, the recognised antigen does not target MPO.
On the other hand, when analysing the data of the 2 new techniques compared to previous ANCA results in the traditional way, it was observed that 36.5% showed positive ANA, and only a small percentage of these showed positive anti-MPO. Again, this could indicate the interference of ANA in reading the ANCA pattern by IIF.
ELISA is used as a traditional confirmatory test for the IIF technique for ANCA, indirect, quantitative and targeted at PR3 and MPO proteins. The recent CytoBead® ANCA kit offers a novel test that covers cells (granulocytes fixed in ethanol) and synthetic microparticles (coated with PR3 and MPO antigens) in the same well, for screening and confirmation of antibodies. Our results found that using this technique38.6% of the samples resulted positive and when confirmation of PR3 was compared with confirmation by ELISA no discrepant results were found. This is considered a very good simultaneous ANCA test alternative using the IIF method without the need for a confirmatory ELISA test, allowing the detection and confirmation of autoantibodies in shorter times, better reproducibility and greater performance in larger samples. This agrees with the study by Sowa et al., who evaluate the efficacy of this new CytoBead® ANCA technique comparing it with the classical tests for ANCA by IIF, PR3 and MPO by ELISA, finding excellent correlation for ANCA PR3 and MPO as well as for P-ANCA and C-ANCA between the classical methods and the multiplex CytoBeads® ANCA.12,27 This is in line with the consensus for handling and interpreting ANCA, and IIF is proposed as a screening and confirmatory test due to its high specificity.15
Unlike the results of CytoBead® ANCA when comparing the data of the L-ANCA® technique with previous ANCA, a discrepancy in the results was observed resulting in low correlation between the 3 techniques. According to these results it can be inferred that the CytoBeads® ANCA technique performs better than the L-ANCA®; although the latter includes simultaneous assessment of ANA, the results were not satisfactory. In the observations of the parent company it is suggested that the presence of ANA could interfere with the interpretation of ANCA. Staining on the cytoplasm or on the lymphocyte surface did not clearly indicate interference by ANA, the staining observed was not sufficiently sharp in some cases. Therefore, if ANA interference was present, ANCA by immunofluorescence could not be interpreted; thus, assessment of lymphocyte staining did not always indicate the presence of ANA, in addition to not detecting similar ANCA results in some patients on the neutrophils observed by the other methods.16
ANCA are currently useful in the diagnosis and classification of vasculitis, however, their usefulness depends on the prevalence in the study population and clinical presentation.5 In rheumatological conditions not associated with vasculitis it is possible to document positivity for ANCA, as in our study, where we found positivity for ANCA in 6.6% of the data related to non-autoimmune rheumatic disease consistent with the presence of ANCA described in different diseases such as primary sclerosing cholangitis, type I autoimmune hepatitis, Felty's syndrome and inflammatory bowel disease. In the latter, positivity is documented in up to 50%–70% of patients with ulcerative colitis and in 10%–30% of individuals with Crohn's disease and hyperthyroidism. However, their usefulness in these scenarios has not been clearly established.26–34
The lack of agreement between clinical data and relevance of ANCA requests in our environment could show the difficulty existing in the different care levels in differentiating the clinical characteristics of vasculitis from other diseases not associated with vasculitis. Thus, in our study, clinical data were obtained from 241 patients, of whom 33% had an initial suspicion of vasculitis, detecting positivity for ANCA in less than 10% of cases, which indicates an overestimation by the health professionals in requesting this diagnostic aid.
Several studies have demonstrated the diagnostic value of ANCA for vasculitis as long as the correct methodology associated with the relevant clinical findings is used. Therefore, ANCA results should always be confirmed by ELISA and it should always be determined whether the patient has positive ANA and ANCA to discount the effect of nucleus staining that generates false ANCA positives and thus make an assertive diagnosis.35–37
Since precise detection for PR3-ANCA and MPO-ANCA has important clinical and pathogenic implications, non-targeted request in the different levels of clinical care and reliable immunoassays for PR3-ANCA and MPO-ANCA have become more widespread every day. The results of this study would support the proposal made by the latest international consensus for ANCA tests in granulomatosis and polyangiitis by means of which testing using specific, high quality methods is suggested as the preferred primary method to support the diagnosis of an ANCA-associated vasculitis. This is without the need to use IIF on first line neutrophils as support; however, further evidence and studies are required to support this recommendation.38,39
ConclusionsANCA are currently useful as an aid in the diagnosis and classification of vasculitis; however, their value depends on the clinical context. Given that the objective was to determine the frequency of ANCA in a population with manifestations of autoimmune disease, the results indicate overestimated requests for this marker as a diagnostic aid in non-targeted primary care consultation.
Secondly, when evaluating the interference of ANA in the interpretation of ANCA, for the adequate assessment of ANCA, the IIF technique should be implemented in laboratories as screening and confirmed with testing for specific anti-PR3 and anti-MPO antigens by any of the commercially available confirmatory assays.
Conflict of interestsThe authors have no conflict of interests to declare.
This study was supported by the Hospital Militar Central and Instituto de Referencia Andino (HMC 2014-080), supported by Biolore Ltda and Química Internacional Ltda.
We would like to thank Sara Pineda-Barragán S. and Laura M. Rey for the technical execution of the autoantibodies and Adela Castro-Gutiérrez for her contribution in the collection of the data.
Please cite this article as: Romero-Sánchez C, Benavides-Solarte M, Galindo-Ibáñez I, Ospina-Caicedo AI, Parra-Izquierdo V, Chila-Moreno L, et al. Frecuencia de ANCA positivos en una población con síntomas clínicos sugestivos de enfermedad autoinmune y la interferencia de ANA en su interpretación. Reumatol Clin. 2020;16:473–479.