Analyse clinical and bone metabolism features in a case series of patients with multiple vertebral fractures after discontinuation of denosumab (DMab).
MethodsAn observational descriptive study analysing data from ten patients with multiple vertebral fractures after DMab discontinuation that were admitted to our rheumatology department between 2015 and 2018.
ResultsThere were a total of 49 spontaneous fractures after an average of 6 DMab doses and 10.9 months from discontinuation. Ninety percent had already received treatment other than DMab 7 of 10 oral bisphosphonates. After discontinuation, CTX and P1NP remained elevated and mean T-score for femoral neck and lumbar spine was lower than before treatment. The most affected vertebrae were L3, L5, D6, D7, D9 and D11.
ConclusionThis report of ten new cases suffering multiple vertebral fractures early after discontinuation of DMab highlights the emerging concern on the subject in the scientific community and the need to clarify its pathogenic mechanism, and to support by solid evidence the new recommendations on its management.
Analizar las características clínicas y de metabolismo óseo de una serie de pacientes con fracturas vertebrales tras la suspensión de denosumab (DMab).
MétodosEstudio observacional retrospectivo de 10 pacientes con fracturas vertebrales tras suspender DMab atendidas en el Servicio de Reumatología de un hospital español de tercer nivel entre 2015 y 2018.
ResultadosSe registraron un total de 49 fracturas espontáneas tras una media de 6 dosis de DMab y transcurridos 10,9 meses desde la suspensión del fármaco. El 90% había recibido tratamiento previo, 7 de 10 bisfosfonatos orales. Tras la suspensión, CTX y P1NP estaban elevados y la media de T-score en cuello femoral y columna lumbar fue menor que previo a DMab. Las vértebras más afectadas fueron L3, L5, D6, D7, D9 y D11.
ConclusiónLa descripción de nuevos casos de fracturas vertebrales múltiples en los meses posteriores a la suspensión de DMab subraya la preocupación emergente en la comunidad científica siendo preciso apoyar en evidencias sólidas las nuevas recomendaciones sobre su manejo.
Denosumab (DMab) is a human monoclonal antibody that acts on RANKL, reducing bone resorption in a reversible way.1,2 It increases bone mineral density (BMD) and reduce reduces bone turnover markers, reducing the risk of fracture in patients with osteoporosis (OP).1–4 Several cases have recently been described of rapid BMD loss and the appearance of multiple fractures following the discontinuation of DMab treatment, leading to a clinical alert and a high level of concern.5–7 Our objective is to analyse the clinical characteristics and bone metabolism of a series of patients with multiple vertebral fractures after discontinuation of treatment with DMab, with the aim of gaining greater knowledge of this medical problem.
MethodsTen women were included who had been diagnosed postmenopausal OP and who had multiple vertebral fractures after discontinuing DMab, and who were treated in the Rheumatology Department of a third level Spanish hospital from 2015 to 2018. The fractures were diagnosed by conventional X-ray imaging, and in some cases magnetic resonance imaging was also used to clarify their chronology. Incidents that scored I or more on the Genant scale were considered to be fractures. Once cases were confirmed their demographic, clinical, analytical and densitometry parameters were studied retrospectively.
Ethical considerations: This study was approved by the Clinical Research Ethics Committee of Hospital Universitario La Paz.
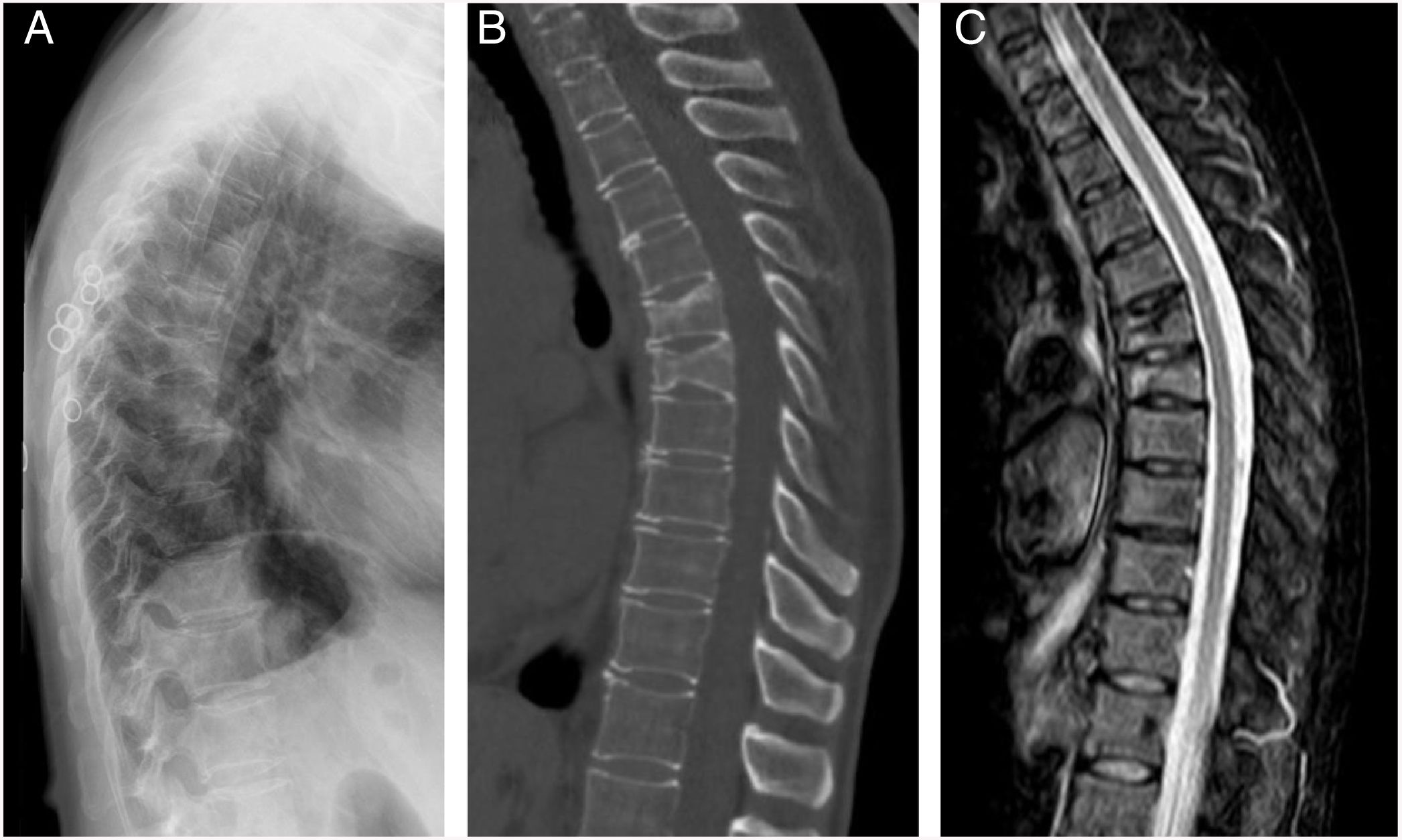
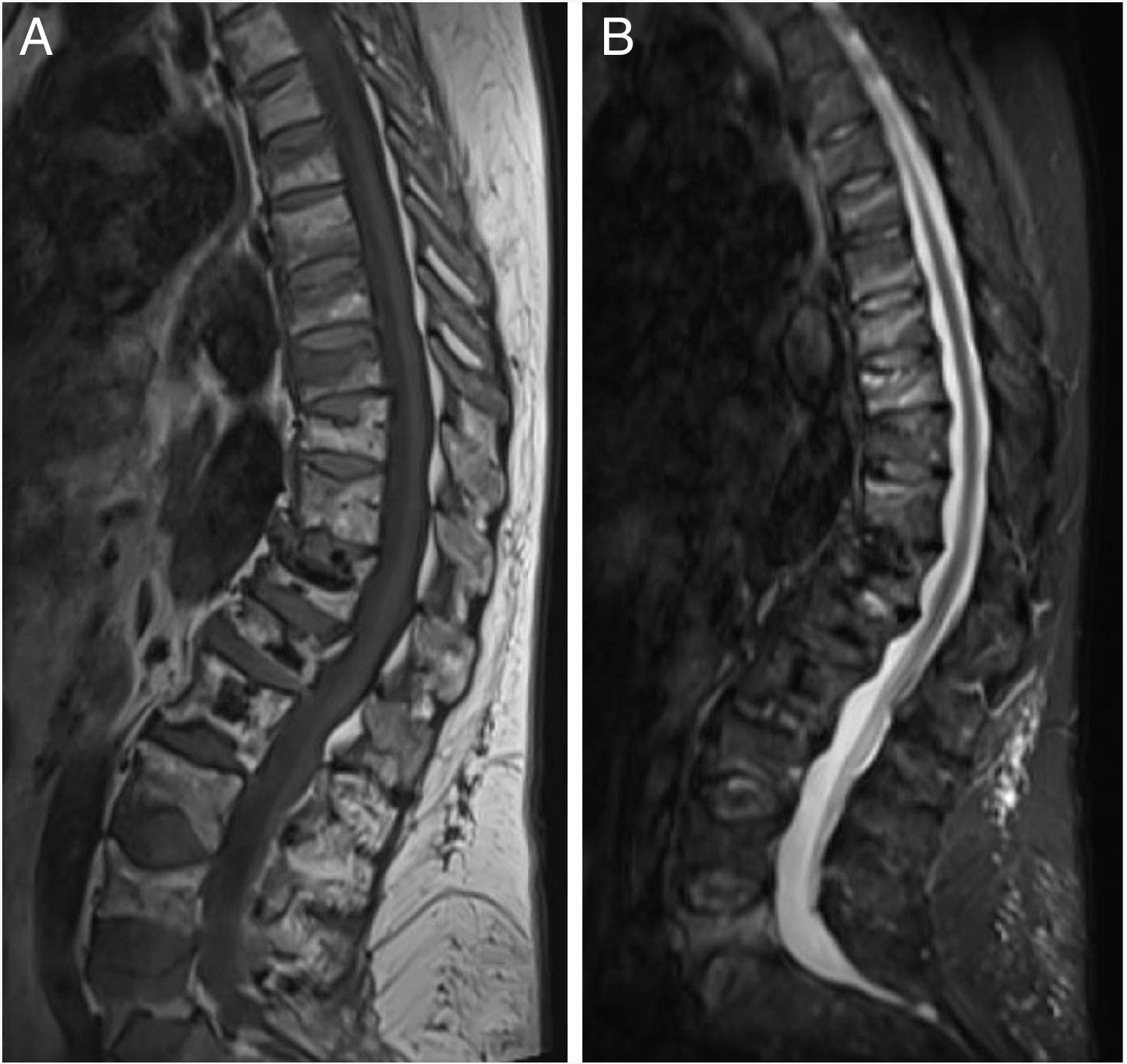
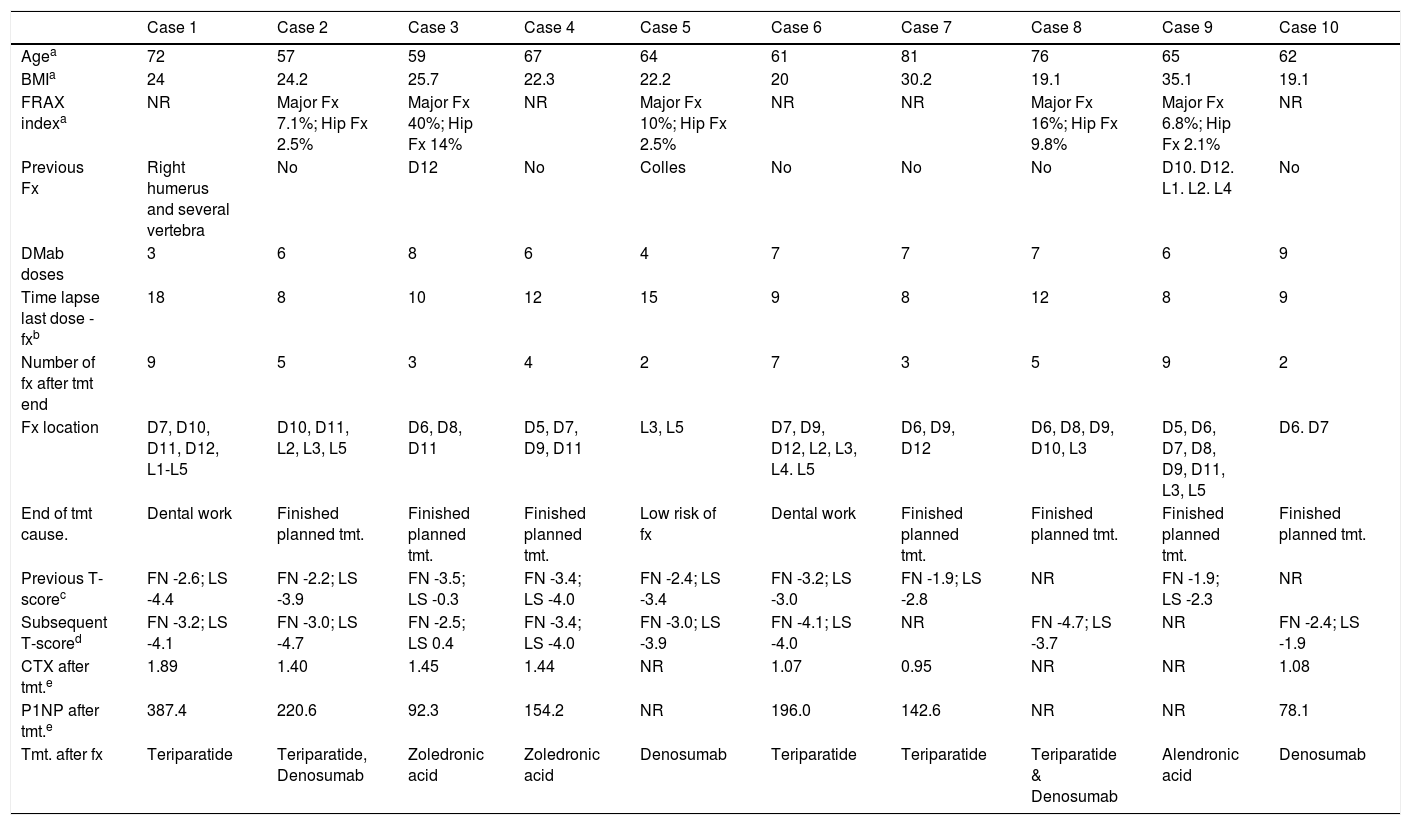
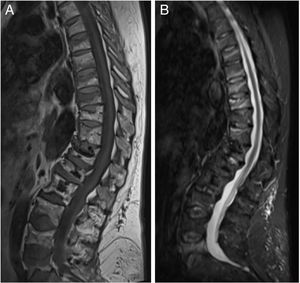
ResultsThe characteristics of the patients are shown in Table 1. Their average age at the start of treatment was 66 ± 7.7 years. Four patients had a previous history of fractures due to fragility, and only one had risk factors for secondary OP (aromatase inhibitors, case 7). Nine patients (90%) had received other treatments prior to commencing with DMab (7 oral bisphosphonates, 5 strontium ranelate, 2 raloxifene, 1 tibolone and 1 calcitonine). The patients received from 3 to 9 doses of DMab, following the dosage recommended in its technical data sheet, with an average of 6 ± 1.7. The reason for withdrawal of the drug was dental work (cases 1 and 6), low risk of fracture (case 5) and termination of the time set by the prescribing doctor. In all cases except two bone density had been measured before starting with DMab. The average T-score before treatment was -2.6 ± 0.6 in the femoral neck (FN) and -3.0 ± 1.3 in the lumbar spine (LS). Likewise, in all of the cases except two (cases 7 and 9) densitometry was performed at least 6 months after the withdrawal of the treatment, with an average T-score of -3.2 ± 0.7 in the FN and -3.2 ± 1.6 in the LS. The bone turnover markers at least 10 months after discontinuation were found to be high, with a collagen telopeptide average (CTX) of 1.32 ± 0.32 ng/ml, and collagen propeptide (P1NP) of 181.6 ± 104.1 ng/ml. The time between the last dose of the drug and the appearance of the first fracture varied from 8 to 18 months, with an average of 10.9 ± 3.3 months. The patients suffered from 2 to 9 fractures, and a total of 49 fractures were recorded. Fig. 1 shows the diagnostic imaging tests corresponding to case 10. In all cases the fractures occurred spontaneously, without associated trauma. The vertebras affected the most often were L3, L5, D6, D7, D9 and D11. Two patients (cases 1 and 2) received vertebroplasty, and new vertebral fractures occurred in both patients. One patient (case 9) commenced taking alendronate six months after discontinuing DMab, following the recommendations of the European Calcified Tissue Society (ECTS), and in spite of this suffered vertebral fractures (Fig. 2). Respecting the pharmacological treatment received after the fractures, the options used the most often were teriparatide (30%), oral bisphosphonates (20%) and DMab (20%).
Patient characteristics.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Agea | 72 | 57 | 59 | 67 | 64 | 61 | 81 | 76 | 65 | 62 |
| BMIa | 24 | 24.2 | 25.7 | 22.3 | 22.2 | 20 | 30.2 | 19.1 | 35.1 | 19.1 |
| FRAX indexa | NR | Major Fx 7.1%; Hip Fx 2.5% | Major Fx 40%; Hip Fx 14% | NR | Major Fx 10%; Hip Fx 2.5% | NR | NR | Major Fx 16%; Hip Fx 9.8% | Major Fx 6.8%; Hip Fx 2.1% | NR |
| Previous Fx | Right humerus and several vertebra | No | D12 | No | Colles | No | No | No | D10. D12. L1. L2. L4 | No |
| DMab doses | 3 | 6 | 8 | 6 | 4 | 7 | 7 | 7 | 6 | 9 |
| Time lapse last dose -fxb | 18 | 8 | 10 | 12 | 15 | 9 | 8 | 12 | 8 | 9 |
| Number of fx after tmt end | 9 | 5 | 3 | 4 | 2 | 7 | 3 | 5 | 9 | 2 |
| Fx location | D7, D10, D11, D12, L1-L5 | D10, D11, L2, L3, L5 | D6, D8, D11 | D5, D7, D9, D11 | L3, L5 | D7, D9, D12, L2, L3, L4. L5 | D6, D9, D12 | D6, D8, D9, D10, L3 | D5, D6, D7, D8, D9, D11, L3, L5 | D6. D7 |
| End of tmt cause. | Dental work | Finished planned tmt. | Finished planned tmt. | Finished planned tmt. | Low risk of fx | Dental work | Finished planned tmt. | Finished planned tmt. | Finished planned tmt. | Finished planned tmt. |
| Previous T-scorec | FN -2.6; LS -4.4 | FN -2.2; LS -3.9 | FN -3.5; LS -0.3 | FN -3.4; LS -4.0 | FN -2.4; LS -3.4 | FN -3.2; LS -3.0 | FN -1.9; LS -2.8 | NR | FN -1.9; LS -2.3 | NR |
| Subsequent T-scored | FN -3.2; LS -4.1 | FN -3.0; LS -4.7 | FN -2.5; LS 0.4 | FN -3.4; LS -4.0 | FN -3.0; LS -3.9 | FN -4.1; LS -4.0 | NR | FN -4.7; LS -3.7 | NR | FN -2.4; LS -1.9 |
| CTX after tmt.e | 1.89 | 1.40 | 1.45 | 1.44 | NR | 1.07 | 0.95 | NR | NR | 1.08 |
| P1NP after tmt.e | 387.4 | 220.6 | 92.3 | 154.2 | NR | 196.0 | 142.6 | NR | NR | 78.1 |
| Tmt. after fx | Teriparatide | Teriparatide, Denosumab | Zoledronic acid | Zoledronic acid | Denosumab | Teriparatide | Teriparatide | Teriparatide & Denosumab | Alendronic acid | Denosumab |
BMI: Body Mass Index; CTX: collagen telopeptide; FN: Femoral neck; Fx: Fractures; LS: Lumbar spine; NR: not recorded; P1NP: Collagen propeptide; Tmt.: Treatment.
This series of clinical cases underlines current concern about the consequences of discontinuing treatment with DMab, given the recent publication of several cases of multiple vertebral fractures a short time after discontinuation. This has been denominated the “rebound effect” or “rebound associated vertebral fractures”.5–7
The characteristics of our series coincide with those of other published series.5–7 An average of 5 vertebral fractures were recorded per patient, all of which were spontaneous and occurred soon after the withdrawal of DMab, from 2 to 12 months after the estimated end of the effect of the drug. BMD data after discontinuation were lower than those detected prior to starting DMab, and bone remodelling parameters were raised. Regarding treatment duration, all of our patients except one had taken DMab during at least 2 years, so that there would seem to be, as has been published beforehand, a relationship between treatment duration and the fall in BMD after discontinuation. As in other series, none of our patients suffered a new non- vertebral fracture, which supports the hypothesis of Anastasilakis et al. that the faster turnover of trabecular bone makes it more sensitive than cortical bone to the increased remodelling that occurs after discontinuation of DMab.5 If fractures do occur vertebroplasty does not seem to be the correct solution, as several cases have been described, as happened with 2 patients in our series (2 and 9, 22.2%), of new vertebral fractures following the said technique.5
When other anabolic drugs are discontinued, such as teriparatide or anti-rebound agents such as oestrogens, there is also an increase in bone remodelling markers and rapid loss of bone, similar to what happens with DMab. Nevertheless, no increase in multiple fractures that can be attributed to the withdrawal of these drugs has been published. As we usually do with patients who cease anabolic therapy with teriparatide, it seems wise to commence therapy with another anti-rebound agent to maintain the BMD obtained with DMab.8,9 Nevertheless, only future studies will be able to show whether this strategy is useful and able to prevent fractures due to fragility described as rebound associated vertebral fractures.
In our series one patient received alendronate 6 months after the discontinuation of DMab, following ECTS recommendations, and she suffered 9 new fractures after this measure. The ETCS recommends starting treatment with another anti-osteoporosis agent when DMab treatment is discontinued: administering a single dose of zoledronic acid or oral bisphosphonate during at least one year.10 Before the publication of these recommendations, patients who discontinued DMab often received no alternative treatment for OP, above all if they had attained their target level of osteopenia without any new fractures. In our series the doctor in charge of the patients considered in 7 cases (7/10, 70%) that they had taken DMab for a suitable length of treatment. With the subsequent acquired knowledge of the efficacy and safety of treatment with DMab and with the FREEDOM extension study data,11,12 it is possible that a current review of the risk of fracture in patients who discontinued treatment would have led us to prolong the treatment with DMab. Nor can we rule out the possibility that the risk of fracture remained high in some of our who treatment had been discontinued. It is relevant that two patients discontinued treatment to undergo dental work, as this centres the problem in the need for suitable anti-rebound discontinuation guides that are shared with stomatologists. The latest SER recommendations for osteoporosis in patients treated with anti-rebound agents who are about to undergo a dental procedure do not recommend discontinuing treatment with bisphosphonates or DMab. If there are risk factors for maxillary osteonecrosis and the surgical procedure will be extensive, the possibility of discontinuation is only considered in the case of the bisphosphonates.13
It has been hypothesised that patients previously exposed to bisphosphonates may undergo a very mild bone turnover rebound after discontinuing DMab. In our series, 70% of the patients had received oral bisphosphonates previously, without any lower incidence of fractures being observed. The implication that sequential therapy with a bisphosphonate before or after treatment with DMab will have an effect when the latter is discontinued would have to be elucidated before changing therapeutic strategy. It would be especially important when DMab is used in young patients who sometimes have no high basal risk of fracture, who are subjected to a temporary risk of BMD reduction, as a therapy with aromatase inhibitors and/or corticoids, and who after the withdrawal of the same may consider the discontinuation of anti-osteoporosis therapy. In these cases the first therapeutic choice may be affected by the adverse effect described.
The existence of previous vertebral fractures, either before or during treatment, was the most consistent prognostic factor for new fractures after discontinuation in previous studies.14 However, in our series only 3/10 patients (30%) had suffered vertebral fractures prior to treatment with DMab, and it two of these patients the fractures were multiple. All of our patients except one (90%) had a BMD in LS and/or FN within the range of OP prior to starting DMab, and this is the risk factor that was identified the most often in our patients.
Our study has limitations: its low number of cases, retrospective observational design and lack of systematisation in radiological, densitometry and bone remodelling marker data gathering. Nevertheless, as the adverse effect is considered to be severe because of its functional repercussions, our experience may underline the need to identify the patients who may be at risk of suffering it, seeking proven therapeutic strategies to prevent the risk of multiple vertebral fractures after discontinuation of DMab.
ConclusionThe description of new cases of multiple vertebral fractures in the months following the discontinuation of treatment with DMab emphasises the emerging concern of the scientific community, so that its pathogenic mechanism (the “rebound effect”) must be elucidated, basing new recommendations on the use of this drug on solid evidence.
Conflicts of interestThe authors have no conflict of interests to declare.
Please cite this article as: Fernández Fernández E, Benavent Núñez D, Bonilla Hernán G, Monjo Henry I, García Carazo S, Bernad Pineda M, et al. Fracturas vertebrales múltiples tras la suspensión de tratamiento con denosumab: serie de diez casos. Reumatol Clin. 2020;16:480–484.