The foot and ankle are common locations of deposition of monosodium urate (MSU) crystals, as indicated by the clinical manifestations presented by patients with gout, which are not limited to the acute inflammation of the big toe. We present a narrative literature review aimed to update the gout involvement of foot and ankle and how it affects the quality of life. Cumulative reports indicate that gout, even at the non-tophaceous stage, could cause pain, gait impairment and limit the mobility at lower limbs. These patients may present difficulties in some activities of daily living such as choosing footwear, thus leading to an impaired quality of life. Gout is a curable disease by dissolving MSU crystals but remains unclear how this could modify some of these foot and ankle manifestations, especially when structural damage has already occurred. Furthermore, a collaboration between rheumatologists and podiatrists seems helpful to understand, relieve these symptoms and improve the quality of life in gouty patients.
El pie y el tobillo son lugares comunes de depósito de cristales de urato monosódico (UMS), como indican las manifestaciones clínicas que presentan los pacientes con gota, que no se limitan a la inflamación aguda del primer dedo del pie. Presentamos una revisión narrativa de la literatura con el objetivo de actualizar la implicación de la gota en pie y tobillo y cómo afecta a la calidad de vida. En la literatura se describe que la gota, incluso en la etapa no tofácea, podría causar dolor, deterioro de la marcha y limitaciones de la movilidad en las extremidades inferiores. Estos pacientes pueden presentar dificultades en algunas actividades de la vida diaria, como la elección de calzado, lo cual implica una calidad de vida deteriorada. La gota es una enfermedad curable si se disuelven los cristales de UMS, pero resulta incierto cómo esto podría modificar algunas de estas manifestaciones en pie y el tobillo, especialmente cuando el daño estructural ya ha ocurrido. Además, una colaboración entre reumatólogos y podólogos sería de utilidad para comprender y aliviar estos síntomas así como mejorar la calidad de vida de los pacientes con gota.
The clinical presentations of any disease that occur more frequently are regarded as typical and prompt a straightforward diagnosis. Conversely, other presentations different to the classical may hamper reach the correct diagnosis and increase the possibility of misclassification. This situation occurs in gout, despite having an immediate and reliable technique – the synovial fluid analysis under the polarized microscope – that provides a diagnosis of certainty.1 The hallmark picture is the acute inflammation of the big toe in a middle-aged man often suffering from renal and cardiovascular comorbidities. Besides the detection of subcutaneous tophi, this presentation poses the highest diagnostic utility compared to the microscopic evaluation.2 However, in general terms, gout diagnoses solely based on clinical data misclassify up to one out of four patients.3 Incorporating the routine microscopic analysis of every synovial fluid sample obtained in clinical practice is a crucial strategy to avoid misdiagnoses. Increasing the knowledge on the clinical spectrum of gout can be useful as well.
The monosodium urate (MSU) crystals deposition occurs in the musculoskeletal (MSK) system, as is demonstrated by the overt manifestations (flares, tophi) suffered by gout patients. However, the deposition and clinical picture probably is more generalized.4 Feet are major MSK sites of MSU crystal deposition, but there are other frequent locations such as knee, wrist, fingers or elbow bursae. In terms of gout flares, old series already reported that big toe and ankle/foot involvement affect to 76% and 50% of gout patients, respectively.5 At the primary care level, 53% of gout flares affected the big toe, while other areas of foot and ankle comprised 18% of episodes. Beyond flares, nonspecific complains focused on feet and ankles are commonly stated by patients with gout in clinical practice, such as persistent walking pain, stiffness or numbness. Subcutaneous tophi are frequently found at Achilles tendon regions, ankle malleoli, lateral aspects of tarsi, over toes (especially the big toes) and even on the plantar surface.
The predilection for feet and ankles of MSU crystals is also confirmed by ultrasound and dual-energy computed tomography (DECT), the imaging techniques that better ascertain the crystal deposition in gout, despite not being interchangeable.6 By ultrasound, reported rates of deposits are 14.2–35.0% in first metatarsophalangeal (MTP) joint, 15.8% in the ankle and 1.6% in the midfoot.7,8 Using DECT, crystal deposits in 1st MTP, midfoot and ankle are seen in 12.1–57.4%, 0.8–7.5%, and 10.8–53.4% respectively.8–12 Prevalence rates vary according to several factors, such as age, disease duration, serum urate (SU) levels or use of urate-lowering agents. Interestingly, crystal deposits were strongly associated with the presence of joint damage such as erosions or joint space narrowing.7,9 Beside joints, imaging techniques also revealed tendon deposition of MSU crystals in feet, with a predominance for Achilles tendons – 26% by ultrasound,13 52% by DECT14 – while other tendons, such as peroneal, extensor digitorum longus, extensor halluces longus, and tibialis anterior, are commonly affected (>10%).
The reason for the predominant involvement of lower limbs, and notably foot and ankle, in gout, remains to be determined. Urate forms MSU crystals when its levels are persistently above the saturation point, that was determined at 6.8mg/dL with a normal body temperature of 37°C.15 At lower values, the saturation level for urate also lowers. Interestingly, an old study proved that the temperature at the big toe is about 35°C,16 thus theoretically facilitating the crystallization of urate.17 Besides temperature and urate concentration, other determinants of the crystallization appear to be the proteins.18 MSU crystals form through templated nucleation, a form of biomineralization that requires the presence of a complementary structure to facilitate a low-energy requiring crystallization. One of the potential protein candidates is the type II collagen, present at both joints and tendons: (i) fragments of proteins containing adhered and well-organized MSU crystals are occasionally seen in the synovial fluid from gout patients19; (ii) the crystal deposition is majoritary on the cartilage surfaces, as demonstrated by arthroscopy20 and ultrasound21; and (iii) at tendons, crystals deposits are mostly seen in the enthesis,14 an area of high mechanical stress for tendon fibers.22 Lower limbs are the leading sites for developing osteoarthritis, as they are weight-bearing. Gout, both as MSU crystal deposition and clinical flares, has been associated with osteoarthritis in some reports,9,23 though not in others.24 Joints affected with osteoarthritis developed cartilage fibrillation, likely facilitating the initial MSU crystal deposition. Afterwards, while hyperuricemia persists, the formation of further MSU crystals continues, now probably using another crystal as the template, as seen in the tophi.18
MethodsFor this narrative review, an electronic search in MEDLINE, Cochrane, Embase and PEDro databases was performed. The search strategy, run at 2019 July 4th, with its used terms, can be found at the Annexed 1. Title and abstracts of retrieved articles were reviewed by all four authors to exclude papers unrelated to the topic of interest. Also, articles known by the authors and others obtained through reviewing the retrieved bibliography were included. The gout nomenclature recommended by the Gout and Crystal Arthritis Network (G-CAN)25 was followed in the writing of this review.
Clinical status of foot and ankle in patients with goutPain, impairment and loss of articular functionality and the decrease in muscular strength are the main signs and symptoms which affect feet in patients with gout.26 These manifestations impact on their daily life activities, measured with HAQ-II; HAQ-II score are considerably reduced during a flare, but they remain low even after the disappearance of the acute symptomatology.27
Pain, disability and impairmentPatients with a history of gout during the intercritical phase present higher levels of foot pain when compared with controls. Although a substantial improvement of pain is achieved through treatment, complete normalization is uncommon, suggesting that foot-related pain in such patients may be a persistent characteristic.26 Pain is mostly located in the 1st MTP joint and the ankle. The presence of pain was reported to be associated with obesity, depression and the occurrence of oligoarticular flares.28
Besides pain, other complaints are frequently referred. Impairment, disability and reduced levels of activity – measured respectively by the Leeds Foot Impact Scale and the Lower Limb Tasks Questionnaire – worsen during flares. Similar to pain, during the intercritical phase of gout, a significant improvement in these scores occurs without achieving a complete recovery. These results are similar to those found in other types of rheumatic diseases such as rheumatoid arthritis,29 again suggesting the impact of persistent low-grade inflammation.
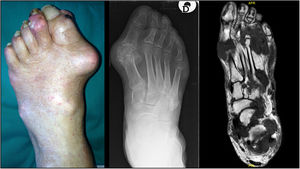
TophiThe presence of tophi in gout indicates chronicity and a lack of effective treatment. They can be found in joints, cartilages, tendons, muscles and periarticular structures.30,31 As stated above, feet are preferred sites for tophi development [Fig. 1], especially in the 1st MTP joints and ankles,27,32 although they may appear in other places of the body such as knees, upper limbs (olecranons, knuckles) or even ears. Presence of tophi in feet has been associated with a decrease in muscle strength32 while some cases of pathological fractures directly related to tophi have been described.33
Although ulceration of tophi is not common, those located in the feet tend to ulcerate more frequently.27,34 Risk factors for ulceration include the number of tophi, their duration, and excessive use of glucocorticoids.34 The presence of small ulcerated areas is common in gout, particularly in cases of chronic polyarticular involvement with tophi. The study by Rome et al.27 performed on seven patients showed the presence of ulcerations on the dorsal side of the middle toes, likely related to unsuitable footwear. Ulcers in patients with gout tend to be highly exudative while carrying with a moderate amount of pain.27 This situation can be worsened by the presence of comorbidities frequently associated with gout such as obesity, type 2 diabetes or peripheral arterial disease, which may delay healing and increase the risk of infection. Tophi ulceration, especially those located in the feet, must be distinguished from infected ulcers of diabetic patients given the frequent association between gout and diabetes.31
Beside ulcers, other skin manifestations are usually present when gout affects superficial synovial structures and joints; this is the case of the podagra, in which peeling often occurs after intense swelling, erythema and edema of the affected joint. When the disease involves deeper joints such as the tibiotalar, dermatological alterations are unusual.
Tendon involvementLower limbs tendons often show MSU crystals deposits at ultrasound and DECT studies.13,14 This involvement occurs mainly, but not exclusively, in tophaceous stages. However, the clinical consequences of the tendon deposition remain unclear. Tenosynovitis and paratenonitis are not frequent manifestations in gout, although there have been reported cases in the patellar, Achilles and peroneal tendons, nearly always associated with tophaceous infiltration.35 Subclinical tenosynovitis of the posterior tibialis has also been observed in asymptomatic individuals with hyperuricemia.36 Tearing of the tendon is also infrequent; however, there are reports of spontaneous tears of the anterior tibialis tendon secondary to a tophaceous gout without trauma and associated in most cases with a forced plantarflexion.37
Limitation of movement, muscular strength and structural alterationsThe range of movement of subtalar joints, both inversion and eversion, as well as dorsiflexion of the 1st MTP joints seems significantly reduced in patients with gout.38 This movement limitation of the joint in the absence of edema or other signs of arthritis suggests a state of subclinical inflammation. It is usually present in patients with intercritical gout. The subclinical inflammation has been proven by analysing the leukocyte count in joints containing MSU crystals.39
Presence of tophi anywhere on foot or ankle is associated with reduced strength of plantarflexion and inversion-eversion of ankles. This association is stronger when the tophus is located in the Achilles tendon.32
As for structural alterations of feet, people with gout are more likely to have hammertoes when compared to a group without gout.38 However, Hallux Valgus is associated with age and gender, but not with the gout characteristics or associated comorbidities.28
Gait characteristicsFew studies analyzed the gait characteristics of gouty patients.29,40–45 However, difficulty in walking during a flare has been considered one of its main discriminatory characteristics. The retrieved studies have focused on the analysis of space-time parameters (STP)29,40–42,44 and the study of plantar pressures.29,40,42,44
The study of STPs consists of analysing the different phenomena observed throughout a gait cycle related to space (step length, stride length and others) and time (such as step time or swing time of the lower limb). Its study has been an area of interest for the different types of inflammatory arthritis, although most studies have been focused on rheumatoid arthritis.40 Concerning gout, statistically significant changes have been found in some of the STPs in patients with gout29,40,42,43 (Table 1). The identified changes vary depending on the reviewed study. In general, patients with gout walk at gait velocity and reduced cadence, as well as with an increase in step time and in the stance phase time. Reports on gait patterns of participants either with and without footwear, as well as those walking at a self-selected29,41–43 or fast speed,41indicate that swing time only increases during fast gait patterns. An only study, which analyzed the walking STPs of patients with gout on footwear in contrast with healthy subjects, has found a significant decrease in the stride and step lengths, and an increase in the stance time with double leg support.29 Equally, when comparing the STPs of patients using footwear which is considered appropriate versus those of patients using their footwear, there is a significant increase in gait velocity, step length and stride length.44 These effects have also been found when comparing the patients’ footwear with that footwear considered deficient, which leads the authors to question whether these findings might be due to other factors.
Changes in spatiotemporal parameters seen in patients with gout.
| Reference | Gait analysis | Disease duration, years, mean | Tophaceous gout (%) | Ancestry | Velocity | Cadence | Step length | Stride length | Support base | Step time | Swing time | Stance time | Single leg support time | Double leg support time |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rome29 | ShoedSelf-selected walking speed | 22.4 | 52% | European 56%Asian 20%Māori 20%Pacific 4% | ↓ | ↓ | ↓ | ↓ | NE | NE | NSSD | NSSD | NSSD | ↑ |
| Stewart41 | BarefootSelf-selected walking speed | 16 | 60% | European 60%Asian 20%Pacific 15%Māori 5% | ↓ | ↓ | NSSD | NSSD | NE | ↑ | NSSD | ↑ | NE | NE |
| Stewart41 | BarefootFast walking speed | 16 | 60% | European 60%Asian 20%Pacific 15%Māori 5% | ↓ | ↓ | NSSD | NSSD | NE | ↑ | ↑ | ↑ | NE | NE |
| Stewart42 | BarefootSelf-selected walking speed | 17 | 71% | European 58%Asian 17%Pacific 21%Māori 4% | ↓ | NSSD | NSSD | NSSD | NSSD | ↑ | NSSD | ↑ | NSSD | NSSD |
| Burke43 | ShoedSelf-selected walking speed | NE | NE | Caucasian 70.1%African American 29.9% | ↓ | NE | NE | NE | NE | NE | NE | NE | NE | NE |
↑: increased; ↓: decreased; NE: not evaluated; NSSD: not statistically significant differences.
Patients with gout also present changes in their peak plantar pressures (PPP) and their pressure-time integrals (PTI).29,40,42 Although the reviewed studies establish different plantar pressure areas, a statistically significant decrease of PPP has been found in the hallux29,42 and the heel42 compared to healthy control groups. Furthermore, a significant increase in PTI values of the midfoot has been noted, being these values lower in the hallux. It has also been observed that PPPs decrease significantly in the third and fifth metatarsal bone when using footwear considered better than the patient's footwear.44 The use of suitable footwear also obtains a significant decrease in PTIs in the lateral and middle side of the heel and the third and fifth metatarsal bone, as well as a significant increase below the midfoot in contrast with use of the patient's footwear.
Reviewed studies show changes in space-time parameters and plantar pressures during gait in patients with gout in contrast with healthy control groups29,40–42 or with the type of footwear used.44 These changes have been linked to a strategy to reduce or prevent pain during walking, which may affect daily life activities. However, despite the correlation among space-time parameters and total scores of Manchester Foot Pain and Disability Index, and the results associated with functional limitation, physical appearance and work/free time, the control over pain is not associated with gait characteristics.41 Nevertheless, it is worth noting that these results were obtained when participants with gout were not experiencing symptoms. For this reason, these adaptations may be explained through an acquired strategy, as a measure to avoid a flare, or as a secondary adaptation to walking as a means of reducing pain.
FootwearPatients with gout present problems when it comes to finding footwear that fits the appropriate shape and with which they feel comfortable, especially during a flare.46 Several studies have identified which characteristics the patients value in footwear46–48; being the most relevant comfort, fit and support. A series of deficiencies associated with footwear has been recognized for consideration at the time of acquiring new footwear. Some of these characteristics are found in the everyday footwear of this group.46–49 Inadequate width and length, high rigidity, weight, cushioning and deficient motion control, lack of fastening elements, lack of heel counter stiffness and high cost are some of them.
Similarly, patients have stated their inability to find footwear that meets an appropriate balance between comfort and appearance in formal or work environments.46,48 They also indicate a lack of confidence when it comes to knowing what footwear they should buy based on previous negative experiences. Patients who use deficient footwear present a higher impairment and limited activity than those who use suitable footwear.47
Specific management of foot and ankle in goutAfter the review of the literature, a few papers were retrieved in relationship with specific treatment of gout in foot and ankle, most of them related to surgical procedures. In 2018, the American College of Foot and Ankle Surgeons, together with the American Association of Nurse Practitioners, performed a consensus about etiology, diagnosis and treatment of gouty arthritis affecting foot and ankle.50 In this consensus, the experts agreed that nonsteroidal anti-inflammatory drugs (NSAIDs) are the first-line therapy for a flare. From the authors’ point of view, NSAIDs are many times contraindicated or not appropriate, while colchicine and corticosteroids (oral or intraarticular) are also effective and safe options. At this consensus, it was also settled the need of urate-lowering therapies, such as allopurinol, to reach the SU target below 6mg/dL. It is also important to mention that the 2016 EULAR treatment guidelines recommend a lower target – <5mg/dL – in severe gout (defined as tophi, chronic arthropathy or with frequent flares) to enhance crystal dissolution.51
Regarding pharmacological treatment, no references dealing with specific management of gout in foot and ankle were found. Nevertheless, it seems reasonable that the treatment would not be different from the ordinary gout patient. Gout must be understood as an MSU crystal deposition disease secondary to hyperuricemia. These MSU crystals are recognized by the innate immune system, causing inflammation.52 The treatment aims to dissolve the MSU crystal storage by achieving a persistent normalization of SU levels. Until MSU crystals are entirely removed, it is also mandatory the flare prophylaxis and, in advance cases, the control of persistent clinical inflammation.
Most of the articles found in the review regarding the targeted management of foot and ankle involvement deal with surgical techniques for cases of tophaceous gout. Interventions can be divided into five types: arthroscopic cleaning, joint replacement, arthrodesis, surgical debridement and graft techniques. In 2016, a systematic review of surgical procedures could not reach a specific recommendation due to the limited good quality studies.53 In addition, a Cochrane review concluded more randomized-controlled trial data for surgical interventions of tophi is needed before drawing firm conclusions.54 One paper assessed the arthroscopic removal of MSU crystals from 1st MTP joints, comparing outcomes against “anti-gout therapy” in 28 patients.55 After more than two years, functional scores and number of attacks significantly favored the surgical group; furthermore, the final SU levels were also lower. Surprisingly, no patient in the control group showed SU levels under 6mg/dL, and the authors did not provide the mean dose of urate-lowering therapy in both groups. Concerning joint replacement, one article reports 16 patients with gout undergoing total ankle arthroplasty, with good results in terms of pain and disability.56 These outcomes were also shown in patients in whom an arthrodesis was performed after tophi excision compared with the excision alone. In fact, in this work, the authors noted that osteoarthritis progressed more frequently in patients without arthrodesis and those with intraarticular tophi.57 Surgical debridement is the classic technique for tophi removal,58 despite being supported only by two relatively recent articles. In the first one, a surgical debridement with a free flap reconstruction in six patients is described with favorable results.59 In the other paper, Lee et al.60 described an intralesional shaving technique focused on reducing the burden of MSU. More recently, a modified bone graft technique called Masquelet's technique was used in situations with significant bone losses, as sometimes occurs in tophaceous gout, with successful results.61
The prescription of footwear considered appropriate is controversial. It produces improvements in pain and foot disability in the short term (2 months),62,63 but eventually, this effect disappears. However, improvements in comfort and fit using suitable footwear tend to persist in time.62 All the studies related to footwear were performed in New Zealand, a fact that needs to be taking into account as could affect data extrapolation.
In the authors’ opinion, the timing and indications for surgical procedures in case of gouty involvement remain to be determined. What is mandatory is to optimize the urate-lowering therapy, because gout is too often poorly managed,64 even by rheumatologists, as it has been demonstrated that only 66% of treated patients reached the targeted SU level.65 Footwear interventions could improve patients’ foot comfortability. Probably in severe gout with considerable structural damage, surgical procedures could be an option, always together with urate-lowering therapies.
ConclusionThe result of the present review highlights that the foot and ankle involvement in gout overtakes the classical picture of recurrent episodes of acute arthritis. Patients affected with gout, even in a non-tophaceous stage, deal with persistent pain and gait impairment. Their ranges of movement at lower limbs are limited, and the choice of footwear may be troublesome for these subjects, especially when tophi are present, all resulting in an impaired quality of life. Focused attention and management appear convenient, where close collaborations between rheumatologists and podiatrists can be constructive. Gout is a curable disease as MSU crystals dissolve when SU levels are persistently normalized; however, the specific effect on these foot and ankle manifestations remains to be determined.
FundingThe present work received no funding.
Conflict of interestMA declares speaking fees and research grants from Grunenthal, Menarini, Astra-Zeneca and Horizon. The rest of the authors declares no conflicts of interest in the making of the present manuscript.
The authors want to thank Mercedes Guerra from Sociedad Española de Reumatología for the assistance with the search strategy.
| (“Gout”[Mesh] OR “Arthritis, Gouty”[Mesh] OR gout[Title/Abstract] OR gouty[Title/Abstract] OR toph*[Title/Abstract] OR tophi[Title/Abstract] OR tophaceous[Title/Abstract] OR podagra[Title/Abstract]) |
| AND |
| (“Foot”[Mesh] OR Foot*[Title/Abstract] OR Footwear*[Title/Abstract] OR Feet*[Title/Abstract] OR Shoe*[Title/Abstract] OR “Foot Orthoses”[Mesh] OR Orthos*[Title/Abstract] OR “Lower Extremity”[Mesh:NoExp] OR “Lower Extremity”[Title/Abstract] OR Gait[Title/Abstract]) |
| NOT (“Clinical Conference”[Publication Type] OR “Congress”[Publication Type] OR “Consensus Development Conference”[Publication Type] OR “Editorial”[Publication Type] OR “Published Erratum”[Publication Type] OR “Letter”[Publication Type] OR “Comment”[Publication Type] OR mice[Title/Abstract] OR mouse[Title/Abstract]) |
| NOT (“Animals”[Mesh] NOT (“Animals”[Mesh] AND “Humans”[Mesh])) |