Hypertrophic pachymeningitis (HP) is a clinico-radiological entity characterized by a thickening of the dura mater that may be focal or diffuse and manifested by a variety of neurological syndromes. Aetiologically, it is classified as infectious, neoplastic, autoimmune, and idiopathic. Many of these formerly idiopathic cases have been shown to fall into the spectrum of IgG4-related disease.
ObjectiveTo describe the case of a patient attended for neurological involvement due to hypertrophic pachymeningitis with initial diagnosis of inflammatory myofibroblastic tumour and final diagnosis of IgG4-related disease.
CaseA 25-year-old woman with neurological symptoms of 3 years’ evolution characterized initially by right hypoacusis, evolving with headache and diplopia. Magnetic resonance imaging (MRI) of the encephalon showed pachymeningeal thickening with involvement of vasculo-nervous structures in the tip of the cerebellum, cavernous sinus, ragged foramen, and optic chiasm. The patient presented for consultation with the result of an incisional biopsy that reported a proliferative lesion combining fibrous elements of fascicular or swirling arrangement with collagenized streaks with dense, lymphoplasmacytic infiltrate and some macrophages, with negative staining for ALK 1, with a diagnosis of inflammatory myofibroblastic tumour.
Due to suspicion of IgG4-related disease (IgG4-RD) the biopsy was sent for review and pertinent complementary studies were requested.
Biopsy reviewNon storiform fibrosis, predominantly lymphoplasmacytic infiltrate, histiocytes, and polymorphonuclear infiltrate in sectors, without granulomas or atypia. Staining for germs negative. Immunohistochemistry with 50–60 IgG4+/HPF cells and range of 15%–20%, CD68+ in histiocytes, CD1a−, S100−.
The patient presented deterioration of visual acuity due to ophthalmic nerve involvement, so glucocorticoid treatment was started in pulses and rituximab with regression of symptoms and imaging improvement of the lesions.
ConclusionHP is a clinical imaging syndrome with variable symptoms and aetiologies that poses a diagnostic challenge.
In this case the initial diagnosis was inflammatory myofibroblastic tumour, which is a neoplasm of variable behaviour, locally aggressive, and can metastasize; it is one of the main differential diagnoses of IgG4-related disease because they share anatomopathological features, including storiform fibrosis.
IgG4-RD is an immune-mediated condition that can have single or multiple involvement. Its diagnosis is complex when it presents with single organ involvement or in non-typical organs (CNS, meninges) in which data are scarce, as in the case of our patient with single organ involvement of the CNS.
Although there are classification criteria to guide non-specialists in the diagnosis, the sum of the clinical picture, imaging, laboratory, pathological anatomy, and immunohistochemistry will always be evaluated together for a definitive diagnosis.
La paquimeningitis hipertrófica (PH) es una entidad clínico-imagenológica caracterizada por un engrosamiento de la duramadre que puede ser focal o difuso manifestada por una variedad de síndromes neurológicos. Etiológicamente se clasifica en infecciosa, neoplásica, autoinmune e idiopática. Se ha demostrado que muchos de estos casos, antes idiopáticos, caen en el espectro de la enfermedad relacionada con IgG4.
ObjetivoDescribir el caso de una paciente asistida en nuestro servicio por compromiso neurológico por PH con diagnóstico inicial de tumor miofibroblástico inflamatorio (TMI) y diagnóstico final de enfermedad relacionada con IgG4.
CasoMujer de 25 años con cuadro neurológico de 3 años de evolución caracterizado inicialmente por hipoacusia derecha, que evoluciona con cefalea y diplopía. Se realiza resonancia magnética nuclear (RMN) de encéfalo donde se evidencia engrosamiento paquimeníngeo con compromiso de estructuras vasculonerviosas en la punta del peñasco, seno cavernoso, agujero rasgado y quiasma óptico. Se presenta a la consulta con resultado de biopsia incisional que informa de lesión proliferativa que combina elementos fibrosos, de disposición fascicular o arremolinada con bandas colagenizadas con infiltrado linfoplasmocitario denso y algunos macrófagos, con tinción negativa para ALK 1 y con diagnóstico de tumor miofibroblástico inflamatorio. Por sospecha de enfermedad relacionada con IgG4 (ER-IgG4) se envía pieza de biopsia a revisión y se solicitan estudios complementarios pertinentes.
Revisión de biopsiaFibrosis de tipo no estoriforme, infiltrado con predominio linfoplasmocitario, en otros cortes se reconocen también histiocitos y polimorfonucleares, sin granulomas ni atipias. Tinción para gérmenes negativos. Inmunohistoquímica con 50–60 células IgG4+/HPF e intervalo del 15 al 20%, CD68+ en histiocitos, CD1a− y S100−. La paciente presenta deterioro de la agudeza visual por compromiso de nervios oftálmicos, por lo cual se inicia tratamiento glucocorticoideo en pulsos y rituximab con regresión de los síntomas y mejoría en resonancia magnética de las lesiones.
ConclusiónLa PH es un síndrome clínico imagenológico con síntomas y etiologías variables que plantea un desafío diagnóstico.
En este caso el diagnóstico de inicio fue tumor miofibroblástico inflamatorio que es una neoplasia de comportamiento variable, localmente agresiva y puede metastatizar; es uno de los principales diagnósticos diferenciales de la enfermedad relacionada con IgG4 debido a que comparten características anatomopatológicas, incluyendo la fibrosis de tipo estoriforme.
La ER-IgG4 es una condición inmunomediada que puede tener afectación única o múltiple. Su diagnóstico es complejo cuando se presenta con compromiso monoorgánico o en órganos no típicos (SNC, meninges) en los cuales los datos son escasos, como el caso de nuestra paciente con compromiso monorgánico de SNC.
Si bien existen criterios de clasificación para orientar a los médicos no especialistas en el diagnóstico, la suma del cuadro clínico, las imágenes, el laboratorio, la anatomía patológica y la inmunohistoquímica serán siempre evaluadas en conjunto llevando al diagnóstico definitivo.
Hypertrophic pachymeningitis (HP) is an inflammation that leads to a diffuse or localized thickening of the cranial dura mater o spinal cord. Patients usually consult with symptoms in connection with the mass effect or focal deficits caused by compression of the nerves or blood vessels. Differential diagnosis is very broad and includes vasculitic disorders (such as granulomatosis with polyangiitis, giant cell arteritis, or Behçet’s disease), immunomediated disorders (rheumatoid arthritis, sarcoidosis), malignity (lymphoma) and infections (tuberculosis [TBC]).1
The group of Wallace et al. believe that disease associated with IgG4 is a common cause of HP.2
We present the case of a patient with cranial HP and involvement of vascular and nerve structures that was thought at first to have a tumoral origin (inflammatory myofibroblastic tumour [IMT]) and was finally diagnosed HP due to IgG4-associated disease.
Rapid diagnosis and correct treatment prevented progression to fibrosis with irreversible symptoms.
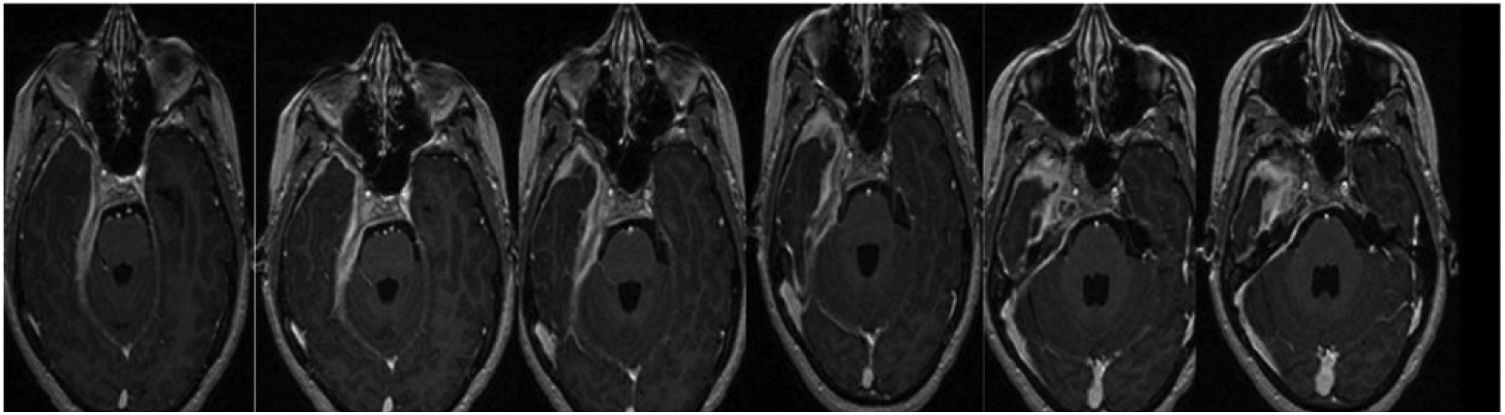
CaseA 25-year-old female patient diagnosed IMT of the base of the cranium consulted in April 2021. She had holocranial cephalalgia, right hypoacusis and subsequently also diplopia that evolved over 3 years. The year before visiting she was hospitalized due to cephalalgia and horizontal diplopia. Physical examination showed involvement of the abducens nerve and right hypoacusis, while magnetic resonance imaging showed evidence of increased ventricular size, occupied mastoid cells and a mass occupying 30mm of intrapetrosal space with increased pachymeningeal enhancement in the base of the cranium (Fig. 1).
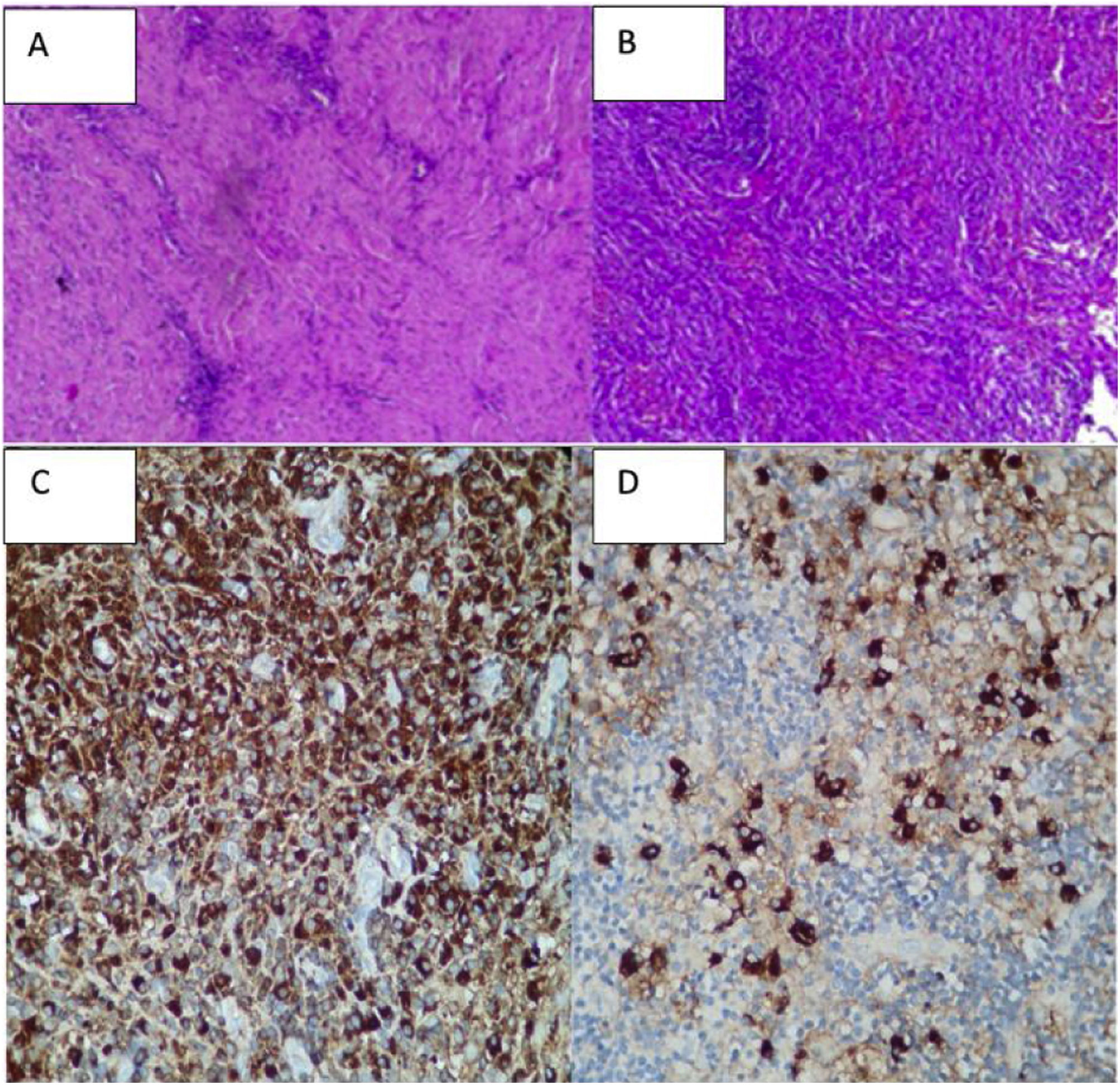
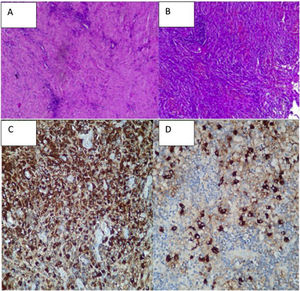
A biopsy was taken of the lesion in January, reporting: proliferative lesion which combines elongated fibrous elements, partially in a spindled or fascicular arrangement and swirled with collagenized bands together with an abundant and dense lymphocytic inflammatory component, dispersed and in accumulations, frequent plasma cells and some macrophages. No necrosis, mitosis or cytologically atypical cells were observed. We interpreted these findings as suggestive of an inflammatory myofibroblastic tumour. Other diseases such as meningioma or cholesteatomatous reaction were ruled out (Figs. 2A and B).
Immunohistochemistry: leukocyte common antigen positive; cytokeratin (AE1/AE3) positive with an epithelial component; smooth muscle actin focal positivity; ALK1 negative.
The immunophenotype found is non-specific and confirms an inflammatory process in soft tissues that may correspond to the diagnostic suspicion of inflammatory myofibroblastic tumour, although the ALK1 marker was negative.
Another NMR imaging scan in April 2021 showed an extensive increase in the expansive process, with post-contrast enhancement that involved the base of the cranium in right parasagittal topography extending towards the carotid canal, jugular foramen, posterior foramen lacerum, homolateral cerebellar tentorium and petrous bone. It also invades the internal acoustic canal, the cavernous sinus and the vertex of the right eye socket, with pachymeningitis at the level of the deep temporal fossa circumferentially surrounding cranial nerve pairs III, V, VI, the cisternal segments and intracavernous nerve, VII and VIII at the level of the internal acoustic canal. Involvement was also detected of the anterior region of the optic chiasm with thickening and post-contrast enhancement that was not seen in the previous study. Defect of partial filling of the homolateral sigmoid sinus probably associated with thrombosis. Supratentorial hydrocephalia persists, with hyperintensity of the subcortical white matter associated with vasogenic oedema.
Positron emission tomography with 18-fluorodeoxyglucose (PET/TC) showed a millimetric focus of activity over the base of the right pterygoid apophysis with a maximum standardized uptake value [SUV]) of 5.5 that fuses slightly asymmetrically with the soft parts of the base of the cranium.
Two weeks later she was admitted to our hospital due to loss of bilateral vision, considered to be caused by neurological involvement by a tumour. Pulses with methylprednisolone were commenced (1g/day/during 3 days).
The biopsy was studied again and an immunohistochemical examination revealed abundant non-storiform fibrosis, between which glandular structures coated in pseudostratified ciliate epithelium without atypical features, which could correspond to trapped native epithelium, and predominantly lymphoplasmacytoid infiltrates.
Histiocytes were also recognised in other slices, as were foci of polymorphonuclear cells (mastoid areas), within the context of prominent endothelial congestive vessels. No granuloma formations or frank necrosis were observed. No increase of eosinophils or obliterating phlebitis was seen (no elastin staining was performed).
Special stainings were used (PAS and Ziehl-Neelsen), without detecting the presence of specific germs.
Immunohistochemistry: all of the controls showed correct reactivity. IgG4: 50–60 plasma cells IgG4+ per high power field. IgG4/IgG ratio: 15%–20% (Fig. 2C and D).
CD68: positive in histiocytes. CD1a: negative. S100: negative (staining in peri-epithelial dendritic cells).
Diagnosis: chronic fibroinflammatory process with additional acute activity.
Note: the lesion had no neoplastic appearance at first, and the abundant fibrous component seems to include native structures without displacing or infiltrating them (non-expansive).
Treatment used methylprednisolone in pulses (1g/day/during 3 consecutive days) after which maintenance therapy consisted of 1mg/kg/day/prednisone and 2 doses of 1g rituximab.
One month after commencing treatment a follow-up NMR imaging scan showed a reduction in the involvement of vascular and nerve structures around the cavernous sinus, the optic chiasm, a reduction in the cerebral parenchymal oedema and reduction in meningeal thickness and enhancement in the base of the cranium.
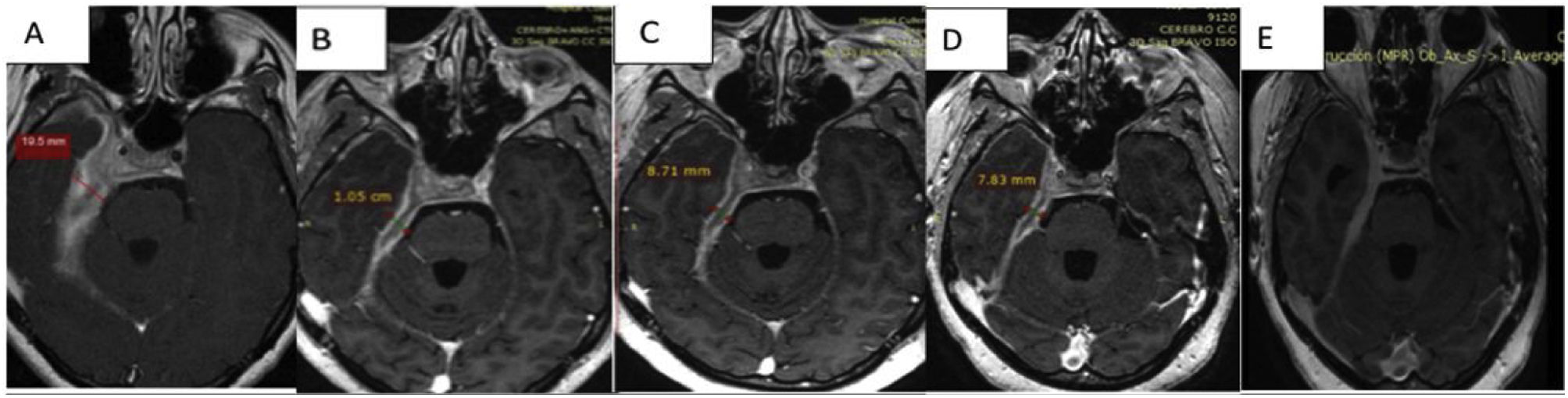
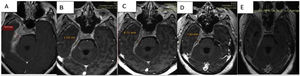
Discussion of the imagesThe successive magnetic resonance imaging studies show pathological tissue that thickens the meninges to 19.5mm, showing an isointense signal in T1, a hypointense signal in T2 and staining with the contrast with moderate restriction of water diffusion.
Right parasagittal topography shows involvement of the base of the cranium extending towards the carotid canal, jugular foramen, posterior foramen lacerum, homolateral cerebellar tentorium and petrous bone. It also invades the internal acoustic canal, the cavernous sinus and the vertex of the right eye socket, with pachymeningitis at the level of the deep temporal fossa circumferentially surrounding cranial nerve pairs III, V, VI, the cisternal segments and intracavernous nerve, VII and VIII at the level of the internal acoustic canal.
The pachymeningitis was found to have progressed, with involvement of the anterior region of the optic chiasm, which was shown by post-contrast enhancement to have thickened. It replaces the right internal jugular vein in its foramen, without finding opacification of the proximal segment of the same. Defect of partial filling of the homolateral sigmoid sinus.
Comparatively, a partial response to the treatment that commenced after the basal study was observed. This study had found a pachymeningeal thickness of 19.5mm; in the follow-up imaging tests this thickness has reduced to 10.5mm (53% of its initial size) one week after the corticoid pulse; to 8.7mm (44% of the initial value) with corticoids plus rituximab; and in the final test 3 months after the start of treatment, with maintenance corticoid therapy of 10mg/day prednisone and methotrexate it measured 7.8mm (40% of its initial size) (Fig. 3A–D).
In the final check-up a reduction in pachymeningeal thickening described in the previous studies was observed, at the level of the chiasma, the internal acoustic canal, the cavernous sinus and the temporal fossa. When slices were performed later after 30min. greater enhancement of the pachymeningeal tissue was observed in comparison with the fibrotic component (Fig. 3E).
Differential diagnosesHP is a syndrome with clinical and imaging test manifestations that is characterized by focal or diffuse thickening of the dura mater, with neurological symptoms that will depend on the location of the lesion rather than its aetiology.1
The causes of pachymeningitis include infections (above all chronic infections such as TBC); autoimmune diseases (such as granulomatosis with polyangiitis [GPA], or neurosarcoidosis); neoplastic causes (primary as in the case of meningioma, or metastasis of liquid tumours such as lymphomas, or solid and most commonly breast tumours); they are classified as idiopathic when the cause of the symptoms is not found, and of these, retrospectively, many would be included in the spectrum of diseases associated with IgG4 (ER-IgG4) described some time after idiopathic HP.
HP is clinically expressed by the compression of vascular and nerve structures. Its most common manifestations are cephalalgia, paralysis of the cranial nerve pairs, endocranial hypertension and venous thromboses, all of which were present in our patient. Although no specific set of clinical symptoms is associated with each aetiology, certain secondary manifestations may guide us in diagnosis. These include respiratory and renal involvement in GPA, adenopathies in sarcoidosis, fibro-inflammatory involvement of other organs (saliva glands, pancreas, adenopathies, etc.) in ER-IgG4; nevertheless, they will be indistinguishable if they debut with isolated involvement of the central nervous system (CNS).
Nuclear magnetic resonance (NMR) imaging with contrast is the method of choice for the study of this syndrome. It shows the diffuse or focal thickening of the dura mater and the involvement of structures, depending on the location.
As our patient was a young woman with supratentorial HP of the base of the cranium, with involvement of vascular and nerve structures, we considered the following aetiologies: GPA, histiocytosis (Langerhans and non-Langerhans), sarcoidosis, a tumoral origin (IMT, meningioma, lymphoma, metastasis), or infections (TBC, fungal) and ER-IgG4.
The first group to be ruled out are the so-called “granulomatous diseases”. Although these entities have a range of aetiologies, they are grouped according to their classical histological characteristics of a granulomatous lesion: histiocytes, macrophages, lymphocytes, multinucleated giant cells and areas of necrosis. They may have an infectious cause such as TBC, an immune system cause like GPA, or an unknown cause such as sarcoidosis.
In GPA the majority of nervous system involvement (up to 50% in different series) affects the peripheral nerves in the form of multiple mononeuritis. The CNS is rarely involved (7%–11%), and it may manifest as cerebral vasculitis, granulomatous meningitis or the involvement of cranial nerve pairs by granulomatous lesions from adjacent structures such as the paranasal sinuses.3 Although meningeal involvement may be found in isolated form (a limited form of GPA), it is usually associated with the paranasal sinuses and granulomatous inflammation is found in the biopsy,4 although these characteristics were not seen in our case.
In sarcoidosis neurological involvement occurs in from 5% to 25% of cases, with a predilection for cranial nerve pairs and the hypophysis. If it affects the meninges, it does so in their innermost layers (leptomeninges). Only 5% of cases will solely involve the CNS when they debut, so that it is fundamental to study the disease systemically. If the CNS is the sole location of the disease, complementary methods such as angiotensin converting enzyme (ACE) and thoracic X-ray will normally be used in the majority of patients; it is useful to study ACE and other parameters in the cerebrospinal fluid.5 In our case the patient only had neurological involvement, with normal extracranial images and ACE, so that this diagnosis was ruled out.
Meningeal involvement by TBC is a severe form of extrapulmonary TBC, and although it is rare in immunocompetent patients, it should always be taken into account in our field. In spite of its infectious aetiology, the progression of this disease may be so slow that diagnosis takes years.6 Our patient is immunocompetent and has no pulmonary involvement (which is the starting point for almost all extrapulmonary TBC).
Within the tumoral diseases a varied group of primary or secondary neoplasias have different degrees of malignity.
The most aggressive diseases, such as solid cancer (breast or lung) metastasis and lymphomas present in acute or sub-acute form, usually with alterations in the general state of the patient, with systemic symptoms and multicentre hypermetabolic lesions. Neoplastic lesions with a low level of aggressivity are at the other extreme, and they include chronic meningiomas and histiocytoses. Two types of meningioma are of especial interest in our case: fibroblastic meningioma (with fibrous tissue and fibroblastic cells) and type that is rich in lymphocytes (with plasma cells and lymphocytes).
Histiocytoses may cause systemic involvement or manifest by involving a single organ; although their prevalence is low, they should very much be taken into account in cases of isolated CNS involvement. They are classified as Langerhans cell histiocytosis (LH) and non-Langerhans (NLH) which include Rosai-Dorfman disease (RDD).
RDD presents clinically with nodal involvement (the lymphatic ganglia), or extranodal involvement (skin, CNS, etc.), both of which are associated with systemic manifestations and analytic alterations (acute phase reagents, anaemia, hypergammaglobulineamia). Its aetiology has not been clearly elucidated, given that cases have been reported with tumoral kinase mutations (BRAF, KRAS, MAP2K1).7 The histological marker of this disease is the sinusal proliferation of histiocytes with emperipolesis (the process in which certain cells transit through the cytoplasm of other cells without being harmed)8 associated with immune markers in which negativity is observed for CD1a together with positivity for CD68 and S100.9 It should be underlined that CNS involvement by RDD does not usual show nodal involvement, which makes it even harder to diagnose. Nevertheless, the cerebral parenchyma is often involved,10 unlike our patient who showed no cerebral parenchyma lesions or alterations in the results of blood analysis.
Our patient had been diagnosed IMT when she consulted, which consists of pseudosarcamatous lesions in different superficial and deep tissues. They may occur in any life stage as formations that occupy spaces; they are characterized histologically by a proliferation of fibroblasts and myofibroblasts mixed with lymphocytes, plasma cells, eosinophils and histiocytes. Nevertheless, the type and combination of the cellular, inflammatory and fibrous components varies from one tumour to another, and even from one field to another within the same tumour.11 Although it has been suggested that IMT and disease caused by IgG4 overlap, as the same elements are involved in both lesions and it is possible that IMT causes storiform fibrosis, there are clearly marked clinical and histological differences between them.
ER-IgG4 affects older patients. Macroscopically IMT consists of a lobulated mass, while lesions secondary to IgG4 appear to be solid tumours, with thickening of the tissues and no defined edges. Histologically IMT is composed of predominantly spindle-shaped myofibroblasts, with lymphocytes and plasma cells. The spindle-shaped cells are arranged in a fascicular pattern or at random within a fibrous stroma with a variable mucoid matrix; the inflammatory infiltrate and fibrosis go unnoticed within the peritumoral tissue. A prominent feature in ER-IgG4 is fibrosclerosis with lymphoplasmacyte infiltration with lymphoid follicles. The fibrosis and inflammatory infiltrate may extend beyond the affected organ. Lymphoid follicles with a germinal centre are found in a subset of IMT (22.7%) and in all IgG4 cases. From 35% to 68% of positivity has been reported for ALK, and it is negative in all cases of ERIgG4. Furthermore, smooth muscle actin staining (SMA) is found diffusely in 100% of the myofibroblastic cells.11
To summarise, these two diseases differ in their immune markers (ALK and SMA in favour of IMT), the number of IgG4+ cells and the IgG/IgG4 ratio, storiform fibrosis and the presence of obliterative phlebitis. It should be underlined that although IMT cases may have a small to moderate presence of IgG4+ cells, the IgG4/IgG ratio is always low.12
Diagnostic summaryER-IgG4+ evolves clinically over time. It commences with non-specific symptoms such as cephalalgia, developing with involvement of the cranial nerve pairs as the lesion spreads in plaques over the meninges, as was shown in repeated magnetic imaging studies. One of the most common sites is therefore hearing involvement, followed by involvement of the cranial nerve pairs which innervate the extrinsic musculature of the eye (III, IV, VI) where they pass through the cavernous sinus, a location which due to its covering is compromised by the pachymeningitis that develops in this zone. Finally, the lesion involved the optic chiasm after evolving over 3 years, so that the patient commenced emergency glucocorticoid treatment before the definitive diagnosis. The diagnosis of HP guided our imaging studies and the final diagnosis. This entity has recently been included within the spectrum of diseases associated with IgG4, so that it has been proposed that many cases of idiopathic HP may be re-categorized as associated with ER-IgG4.2
Analysis of the biopsy showed a high number of IgG4+ cells (50–60/high power field) and a range of from 15% to 20% with some histiocytes and neutrophils, with negative immune markers for ALK1 and S100, focally positive for CD68 and SMA. Based on this neoplastic and granulomatous diseases were ruled out, as the findings showed nothing atypical, mitotic figures, the formation of granulomas or necrosis. Histiocytosis was also ruled out as there was no preponderance of histiocytes and there were no foam cells or giant cells; we should particularly take into account the fact that extranodal RDD has more lymphoid follicles with germinal centres, fibrosis, fewer histiocytes and less emperipolesis, all of which are attributable to ER-IgG4. No large histiocytes or emperipolesis were identified in our case, and nor was specific staining for S-100 protein.
The diagnosis of ER-IgG4 limited to pachymeningitis was supported by the symptoms and clinical signs, suggestive histological data and the response to treatment (corticoids and rituximab).13 This case was considered to be a presentation of ER-IgG4 in a single organ, as described previously in the pancreas and many other organs.14,15
In single-organ ER-IgG4, the level of IgG4 may rise or remain within normal limits, and in connection with this a positive correlation has been found between IgG4 in serum and the number and severity of the organs involved.16–18 In fact, a high level of IgG4 in serum is not an absolute criterion for ER-IgG4, as it seems to be more of a marker of systemic disease. Moreover, in the complete diagnostic criteria for ER-IgG4, disease associated with IgG4 may be considered without taking into account levels of IgG4 in serum.19 On the contrary, a higher-than- normal value does not confirm the disease, given that it may also rise in other diseases.20,21
Outstanding points in HP caused by ER-IgG4Wallace et al. reviewed the pathological anatomy of patients with non-infectious HP during the last 25 years. Fourteen patients fulfilled the histological and immunohistochemical criteria. Four cases (66.6%) considered to be idiopathic HP were diagnosed ER-IgG4, representing 29% of the cases reviewed. The others were: 3 patients (21%) granulomatosis with polyangiitis, 2 (14%) lymphomas, and one case each of rheumatoid arthritis, giant cell arteritis and sarcoidosis. Two patients (14%) remained without a diagnosis and were considered to be idiopathic HP. No single study, clinical test, serology, cerebrospinal fluid or radiology alone could identify the cause of HP. A biopsy and histopathological and immunohistochemical studies are necessary to reach the correct diagnosis.2
In the Revista Cubana de Reumatología a Latin American group of rheumatologists presented 11 cases of ER-IgG4 with pachymeningeal involvement. The first clinical manifestations were cephalalgia, hypoacusis and involvement of cranial nerve pairs. Eight of the 11 patients had single organ involvement, and 2 of 8 patients had a high level of IgG4 in serum.22
Lu et al. observed that the most common first symptoms of HP associated with IgG4 (33 cases) were cephalalgia in 22 cases (67%), 11 cases (33%) with cranial nerve paralysis, and 7 cases (21%) with sight problems (diplopia or a reduction in visual acuity). The majority of the patients also had other signs and symptoms of systemic disease, although 10 (30%) only had (single organ) pachymeningeal involvement.23
We should be aware that this is a new disease with a variable clinical spectrum, so that diagnosis is difficult and in not a few cases it goes unnoticed.
Three agreements on classification criteria are used to standardize such a varied population and study the disease (Desphande et al.,24 Umehara et al.25 and Wallace et al.26).
It is firstly important to underline that these are classifications rather than diagnostic criteria, so that their use is restricted to scientific works and providing a guideline for doctors who are not specialists in this area to help diagnose the disease.
Regarding the agreement of Desphande et al.,24 it should be pointed out that the anatomic and pathological characteristics taken into account for diagnosis are not useful in organs which have only been covered in a few communications (the meninges, CNS and the skin). From a histological point of view, the presence of histiocytes, macrophages and neutrophils does not exclude the diagnosis, as they have to be found to be predominant. This group proposes different cut-off points for each affected organ, which for the meninges is 10 IgG4+ cells per high power field. Although this criterion was met by our patient, it was established on the basis of a series of patients from a small sample.27
Our patient did not fulfil the criteria set by Umehara et al.25 because she did not have more than 135mg/dl IgG4 in serum or a ratio of IgG4/IgG cells higher than 40%. Nevertheless, these authors propose that in patients with a serum concentration of IgG4 lower than the cut-off value (135mg/dl) and single organ involvement, as in our case, the IgG4/IgG ratio in plasma may be taken into account. Although this agreement does not give a cut-off point for the ratio in serum, this same group analysed the same and reached the conclusion that a ratio in serum higher than 8% would have at least the same sensitivity and specificity as IgG4 in serum higher than 135mg/dl.28 Our patient had total IgG4 in serum of 118mg/dl, but with a ratio in serum of 10.5%. It should be pointed out that one third of patients may have a normal level of IgG4 in serum, above all those with single organ involvement, those treated beforehand with steroids, and particularly in cases that involve the pachymeninges.29
Respecting the cell interval in pathological anatomy we should remember that it may vary from organ to organ, and it depends on the phase the disease is in. Thus, in cases of prolonged evolution the number of IgG4+ cells fall, and fibrosis may not show the typical storiform pattern known as the “burnout” phase.30
Lastly, in connection with the EULAR/ACR agreement (Wallace et al.),26 although our patient had histiocytes and neutrophils, the latter were not predominant but rather were isolated, so that the case did not fulfil this exclusion criterion. In terms of the addition of points there was dense lymphoplasmacytic infiltrate (4 points) and immunohistochemistry with 60 IgG4 cells and a 20% ratio that assigned a weighting of 7 additional points, giving a total of 11 points. Although it therefore does not have the weighting assigned to the disease by Wallace et al., as the presence of pachymeningitis is not exclusive to ER-IgG4 it is not weighted.
With respect to the measurement of IgG4 in cerebrospinal fluid, a study compared IgG subclasses in the cerebrospinal fluid of patients with pachymeningitis associated with IgG4 with those patients with other inflammatory, infectious and neoplastic forms of pachymeningitis. Additionally, the researchers calculated the IgG4 index and IgG4Loc values to evaluate the degree of blood-brain barrier permeability and the intrathecal production of total IgG and its subclasses.
Compared with healthy controls and patients with other forms of HP, patients with IgG4-related HP showed higher concentrations of IgG4 in the cerebrospinal fluid, as well as higher levels of IgG4 and IgG4Loc values. The latter figure, which calculates and integrates IgG4 concentrations in serum and cerebrospinal fluid in association with the blood-brain barrier, has been shown to have better diagnostic performance in differentiating both groups. IgG4Loc values higher than 0.47 had 100% sensitivity and specificity in differentiating IgG4-related HP from other causes of inflammatory HP.29,31–33
Respecting the use of PET/CT with 18-FDG in this entity, we know how important this is when determining not only the degree of active inflammation in the meninges, but also when identifying extracranial meningeal involvement and the involvement of other organs by the disease, and when evaluating the response to treatment.34 Difficulties arise in the latter when assessing brain lesions. It would therefore be advisable to use a different radio tracer, 11C methionine, due to its low uptake in normal brains (MET/PET).35
It was not possible to measure the level of IgG4 in the cerebrospinal fluid or the IgG4LOC index or to use PET with methionine in this patient.
Single organ involvement, in this case the pachymeninx, may be a problem for diagnosis. Although the level of IgG4 in the cerebrospinal fluid, IgG4LOC and PET/CT with methionine were not performed in our patient, these may play an important role in the study of these patients.
ConclusionSingle organ involvement by ER-IgG4 is hard to diagnose. In our case the sum of the clinical manifestations, images, pathological anatomy with immune markers and the good response to treatment gave rise to the clear diagnosis of HP due to ER-IgG4.
We underline that it is fundamental to interpret the histology within the context of suitable clinical and imaging tests, using the diagnostic criteria for the disease as a guide which should be individualized for each case studied.
A reliable diagnosis will rarely be based solely on pathological findings.
FinancingThe authors declare that they received no financing for this work.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank Dr. Fernando Martinez Valle for revising the manuscript.