Biologic therapies with tumor necrosis factor (TNF)-α inhibitors are widely used to treat inflammatory diseases, such as rheumatoid arthritis (RA) and spondyloarthropathies (SpA).1 They are administered subcutaneously (SC) or intravenously (IV), and the route has an influence on their bioavailability. In SC administration, there are 2 aspects to consider: the site and the injection technique,2–5 both of which are key factors in the proper administration of the injections; thus, training of patients by the nursing staff is essential for SC self-administration of anti-TNF agents.
The thickness of the subcutaneous tissue (ST) can influence the proper distribution of the drugs throughout the organism. This thickness can be affected by age, sex and body mass index (BMI),2,4–6 variables that are important for the determination of the administration site and technique for each patient. In the case of insulin-dependent diabetes mellitus, the needles for self-administration devices are available in a wide variety of lengths, depending on the ST thickness, as has been reported previously in a number of publications. However, there are no studies on this subject dealing with rheumatic diseases treated by SC administration of anti-TNF agents. The proper injection technique ensures that the SC anti-TNF agent be injected into ST, rather than intramuscular (IM) or intradermal tissue.
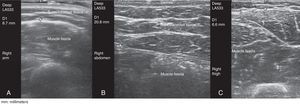
This prospective cross-sectional observational study involved 117 patients with RA (n=59) or SpA (n=58) being treated with an anti-TNF agent that the patients administered SC to themselves for a minimum of 6 months. The thickness of the ST was measured in all the patients at the sites recommended for SC injection (arms, abdomen, thighs), regardless of the preferred site for self-administration, using gray-scale ultrasound (ultrasound system equipped with a 6–18-MHz multifrequency linear transducer). Ultrasound measurement of the ST was always performed using the same method and with the patient in the sitting position. Gel was applied between the probe and the skin to avoid putting pressure on the ST with the probe (Fig. 1). The probe was placed transversely and longitudinally, on the right and left sides, at the sites recommended for SC injection. We then calculated the average between the two measurements.
The results are expressed as the mean±standard deviation (SD) for continuous variables and as absolute frequencies and percentages for categorical variables. The continuous variables in independent groups were compared using the independent samples t-test when 2 groups were involved. For the comparison of 3 groups, we used 1-way analysis of variance (ANOVA) with the Tukey test, or the Kruskal–Wallis test with the Mann–Whitney test and the Bonferroni correction to determine the unpaired samples, depending on the assumption or rejection, respectively, of the null hypothesis. The linear relationship between independent variables was established by means of the Pearson correlation coefficient. Fisher's exact test of independence for categorical variables was applied in the case of 2 dichotomous variables, and the chi-squared test when any of the variables had more than 2 categories. Logistic models were developed as dichotomous outcomes of clinical remission or no clinical remission, according to the Disease Activity Score for 28 joints (DAS28) and C-reactive protein (CRP) level. We analyzed age, BMI, ST thickness in abdomen, arms and thighs, time since diagnosis, time since initiation of anti-TNF therapy (with etanercept, adalimumab, others [because of the small sample size, patients treated with golimumab and certolizumab pegol were analyzed jointly]), and concomitant treatment with disease-modifying antirheumatic drugs. The odds ratio was calculated with its 95% confidence interval). P values ≤.05 were considered to indicate significance. The statistical analyses were performed with the SPSS statistical software package (v15.0).
Due to the small sample size, our RA and SpA patients were analyzed jointly, according to the anti-TNF agent utilized. Fifty-nine patients (50.5%) had been diagnosed with RA and 58 (49.5%) with SpA. Fifty-six patients (47.9%) were receiving etanercept; 52 (44.4%), adalimumab; 7 (6%), golimumab; and 2 (1.7%), certolizumab pegol. Eighty-two (70%) self-administered the anti-TNF agent in abdomen, 23 (19.7%) in thigh and 12 (10.3%) in arm.
The majority of the patients were women (n=61, 52.1%). The mean±SD (range) for the variables were as follows: age, 52.77±13.28 (24–82) years; weight, 74.01±15.19 (46–125)kg; height, 1.65±0.08 (1.43–1.84)m; and BMI, 27±4.75 (18.44–41.58)kg/m2. The clinical response to the anti-TNF agent was evaluated using the following remission or activity criteria: DAS28 and CRP for the patients with RA and the Ankylosing Spondylitis Disease Activity Score (ASDAS) for the patients with SpA. In RA, remission was considered to be indicated by a DAS28<2.6 and no remission by a DAS28≥2.6, and remission in SpA by an ASDAS<1.3 and no remission by an ASDAS≥1.3.
The mean thickness of the ST was significantly greater in abdomen (mean±SD, 24.7±14.3mm) than in thigh (11.6±4.9mm) or in arm (9.1±4.5mm) (P<.0005).
The injection site was significantly associated with clinical disease activity measured by DAS28-CRP/ASDAS. The percentage of patients with active disease was significantly higher among those who self-administered the anti-TNF agents in arm (n=9; 75.0% of the patients) than in those who self-administered the treatment in abdomen (n=33; 40.2% of the patients) or thigh (n=4; 17.4% of the patients) (P=.004).
The ST thickness may be an important factor in the selection of the needle length and injection technique,7–9 to ensure that the drug is administered to ST rather than IM tissue,8,10 which, in turn, influences the therapeutic response. High-frequency ultrasound enables direct measurements of ST thickness at any anatomical site and is harmless, easy to perform and rapid. Our results showed a significantly lower percentage of remission in the group that self-administered the anti-TNF agent in arm (n=12), with a significantly thinner ST layer (ST thickness in arm; mean±SD, 7.5±2.3mm).
We propose ultrasound measurement of ST thickness in patients who are to begin anti-TNF therapy or undergo a weight change once being treated, to ensure the correct selection of the injection site.
Conflicts of InterestLara Valor has received speaking fees from AbbVie, Roche Farma, Bristol-Myers Squibb and Pfizer.
Inmaculada de la Torre is a European physician who has been working for Eli-Lilly & Co. since February 2013.
Esperanza Naredo has received speaking fees from AbbVie, Roche Farma, Bristol-Myers Squibb, Pfizer, UCB Pharma, General Electric Healthcare and Esaote. She has also received research funding from UCB Pharma and Merck Sharp & Dohme.
We wish to express our appreciation for the participation of all the patients, without whom this study would not have been possible. We also thank Jesús Garrido for his help with the statistical study.
Please cite this article as: del Río T, Valor L, de la Torre I, Naredo E. Impacto del grosor del tejido celular subcutáneo en el sitio de la inyección medido por ecografía sobre la respuesta terapéutica a fármacos antifactor de necrosis tumoral subcutáneos. Reumatol Clin. 2016;12:300–301.