To describe the evolution of lung function in a cohort of rheumatoid arthritis (RA) patients with interstitial lung disease (ILD) treated according to the medical judgment of attending physicians.
MethodsRetrospective cohort of RA patients with ILD, defined by a restrictive pattern in lung function tests and evidence of ILD in high resolution computed tomography (HRCT). Patients had an assessment of lung function including spirometry, diffusing capacity for carbon monoxide (DLCO), and HRCT. At a minimum of 4 months of follow-up, a second assessment of lung function was done. All patients received a high dose of prednisone (1mg/kg/day) scheme for 6 weeks with a reduction scheme ending with a dose of 10mg/day of prednisone at about 6–8 months of follow-up. Methotrexate was used in 18/40 (45%) patients and leflunomide or azathioprine or both were indicated in 22/40 (55%).
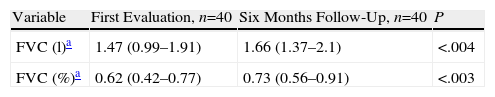
ResultsForty patients were identified. An indeterminate pattern with diffuse ground glass and reticulation images (50%) was the most prevalent pattern on HRCT scans. At a minimum of 4 months of follow-up, an improvement in basal FVC values was observed (median (IQR)) 1.47 Lts. (0.99–1.91) vs 1.66 Lts. (1.37–2.1)), P<.004. Patients with lower Kazerooni scores for fibrosis (<0.47) had a better improvement in the FVC values.
ConclusionsPatients with RA and ILD may have an improvement in the FVC after a treatment with high doses of corticosteroids and disease modifying antirheumatic drugs (DMARDs).
Describir la evolución de la función pulmonar en una cohorte de pacientes con enfermedad pulmonar intersticial asociada a la artritis reumatoide (EPI-AR), tratados de acuerdo al juicio de sus médicos tratantes.
MétodosEstudio de cohorte retrospectivo de pacientes con EPI-AR, demostrada con un patrón restrictivo en las pruebas de función pulmonar, y de enfermedad pulmonar intersticial en las tomografía de alta resolución (HRCT). Los pacientes tuvieron una evaluación basal de la función pulmonar que incluyó espirometría, DLCO y HRCT. En un mínimo de 4 meses, una segunda evaluación de la función pulmonar fue realizada. Todos los pacientes recibieron una dosis alta de prednisona (1mg/kg/día) por 6 semanas con un esquema de reducción, con una dosis de prednisona de 10mg/día a los 6 u 8 meses de seguimiento. Se prescribió metotrexate en 18/40 (45%) pacientes, leflunamida o azatioprina, o ambas en 22/40 (55%) pacientes.
ResultadosSe identificaron 40 pacientes con EPI-AR, El patrón más frecuente en la HRCT fue el indeterminado, con imágenes reticulares y de vidrio despulido en un 50% de los casos. A los 4 meses de seguimiento se observó una mejoría de los valores de la capacidad vital forzada (CVF), mediana basal de 1.47 Lts, intervalo inter cuartil (IIC): 0.99-1.91 Lts., Vs. Mediana de 1.66 Lts., IIC:1.37-2.1 Lts., p<0.004. Los pacientes con los puntajes menores de la escala de Kazerooni para fibrosis pulmonar, (< 0.47) fueron los que presentaron una mejoría en los valores de la CVF.
ConclusionLos pacientes con EPI-AR pueden tener una mejoría en la CVF después del tratamiento con dosis altas de corticosteroides y fármacos modificadores de la enfermedad. (FARMES).
Rheumatoid arthritis (RA) is a systemic inflammatory autoimmune disease that can cause interstitial lung and airway disease.1 Lung involvement confers a poor prognosis and it is an important cause of death in RA patients.2,3 A cohort study described that the cumulative incidence for interstitial lung disease (ILD) in RA is 7.8%.4 Nowadays, there is no consensus of how patients should be treated. It has been proposed that the American Thoracic Society/European Respiratory Society (ATS/ERS) classification for idiopathic interstitial pneumonias should be used to classify RA patients with interstitial lung disease, and there is evidence that the tomographic pattern of lung disease may be an important prognostic factor.5–8 This study was done with the aim of describing the evolution of lung function at 6 months of follow-up in a cohort of RA patients with ILD, treated according to the medical judgment of the attending physician and to describe if there is a difference in the response to treatment according to the tomography findings.
MethodsThis is a retrospective cohort of RA patients classified according to the ACR/87 criteria,9 all with ILD defined by a restrictive pattern in lung function tests (TLC <80%, normal FEV1/FVC and FVC <80%) and with evidence of ILD in high resolution computed tomography (HRCT) and a low diffusing capacity for carbon monoxide (DLCO). Patients were evaluated and treated in the interstitial lung diseases unit at the Instituto Nacional de Enfermedades Respiratorias, a national referral center for respiratory diseases. Attending physicians (pulmonologists and rheumatologists) have experience in the medical assessment of ILD. At baseline patients have an evaluation of lung function including spirometry, DLCO, and HRCT. Then, at a minimum of 4 months of follow-up, a second assessment of lung function was done.
A complete description of baseline HRCT findings was done. HRCT findings were classified according the ATS/ERS criteria. The HRCT reader was blinded to clinical data. A Kazerooni scale for fibrosis and inflammation was also calculated.10 Our reader has a high intraobserver concordance in the Kazerooni score, with an intraclass correlation coefficient of 0.90 (95% CI: 0.84–0.94). HRCT was performed with 1.0 or 1.5mm thick axial section taken at 1cm intervals throughout the entire thorax and were reconstructed using a high-spatial frequency algorithm. Between 20 and 25 CT scans images were acquired in each patient. The study was approved by the Institutional Review Board of our institute. As this is a retrospective study, no written informed consent from patients was taken.
Statistical AnalysisPulmonary function tests values were compared at a 6-month of follow-up with baseline values using the Wilcoxon signed-rank test or the t-test for paired data. α was set at 5%, all analyses are two sided.
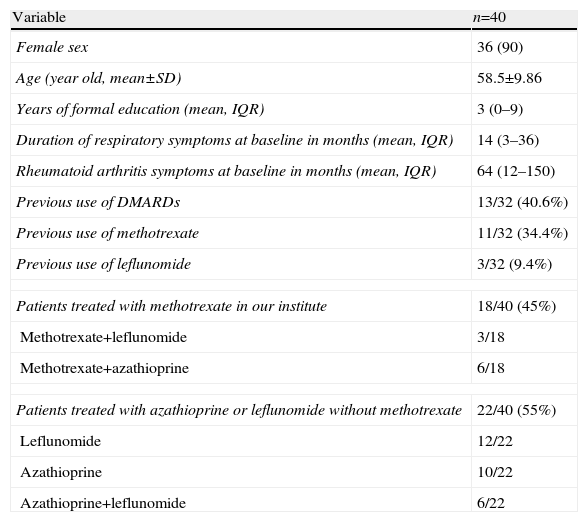
ResultsForty patients with ILD related to RA were identified, 90% were female with a mean age of 58.5±9.86 years. Respiratory symptoms at diagnosis had a mean time since onset of 13 months. The majority of patients had a longstanding RA, with a mean time with joint symptoms of 64 months (IQR 12–150); only 40.6% of patients used disease modifying antirheumatic drugs (DMARDs) before the diagnosis of ILD related to RA. The rest of demographic data and the clinical characteristics of the patients are presented in Table 1.
Patients Included in The Study.
| Variable | n=40 |
| Female sex | 36 (90) |
| Age (year old, mean±SD) | 58.5±9.86 |
| Years of formal education (mean, IQR) | 3 (0–9) |
| Duration of respiratory symptoms at baseline in months (mean, IQR) | 14 (3–36) |
| Rheumatoid arthritis symptoms at baseline in months (mean, IQR) | 64 (12–150) |
| Previous use of DMARDs | 13/32 (40.6%) |
| Previous use of methotrexate | 11/32 (34.4%) |
| Previous use of leflunomide | 3/32 (9.4%) |
| Patients treated with methotrexate in our institute | 18/40 (45%) |
| Methotrexate+leflunomide | 3/18 |
| Methotrexate+azathioprine | 6/18 |
| Patients treated with azathioprine or leflunomide without methotrexate | 22/40 (55%) |
| Leflunomide | 12/22 |
| Azathioprine | 10/22 |
| Azathioprine+leflunomide | 6/22 |
Medical treatment was decided by the attending physician. A myriad of treatments were used, nevertheless all patients received a high dose of prednisone (1mg/kg/day) scheme for 6 weeks with a reduction scheme ending with a dose of 10mg/day of prednisone at about the 6–8 months of follow-up. DMARDs used are described in Table 1. Methotrexate was used in 18/40 (45%) patients and leflunomide or azathioprine or both were indicated in 22/40 (55%). At a minimum of 4 months of follow-up (mean of 10.5 months of follow-up, IQR: 6–18 months of follow-up) an improvement in baseline FVC values was observed (mean (IQR) 1.47 Lts. (0.99–1.91) vs 1.66 Lts. (1.37–2.1)), P<.004 (Table 2). No differences were found between the DMARDs used. Patients had a mean 22.5 months of follow-up after the baseline evaluation. Four patients died, one of right heart failure, two of acute exacerbations and one died in an outpatient setting, possibly of respiratory failure. The mean survival was 64 months. No differences were found between DMARDs and survival.
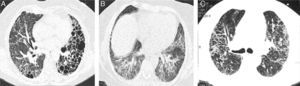
We had the initial HRCT of 33 patients; of these, 4 (12%) had a usual interstitial pneumonia (UIP) pattern, 12 (38%) had nonspecific interstitial pneumonia (NSIP) pattern and 17 (50%) had an indeterminate pattern with diffuse ground glass and reticulation images (Figs. 1 and 2). The mean of the Kazerooni fibrosis scales was 0.47 and the mean of the Kazerooni ground glass scale score was 2.33 (Table 3). We compared the response in the FVC at 6 months of follow-up between patients with a value <0.47 of the fibrosis Kazerooni scale and those who had higher values. Those with lower Kazerooni scores (<0.47) were the ones who had a significant improvement in the FVC values (Table 4).
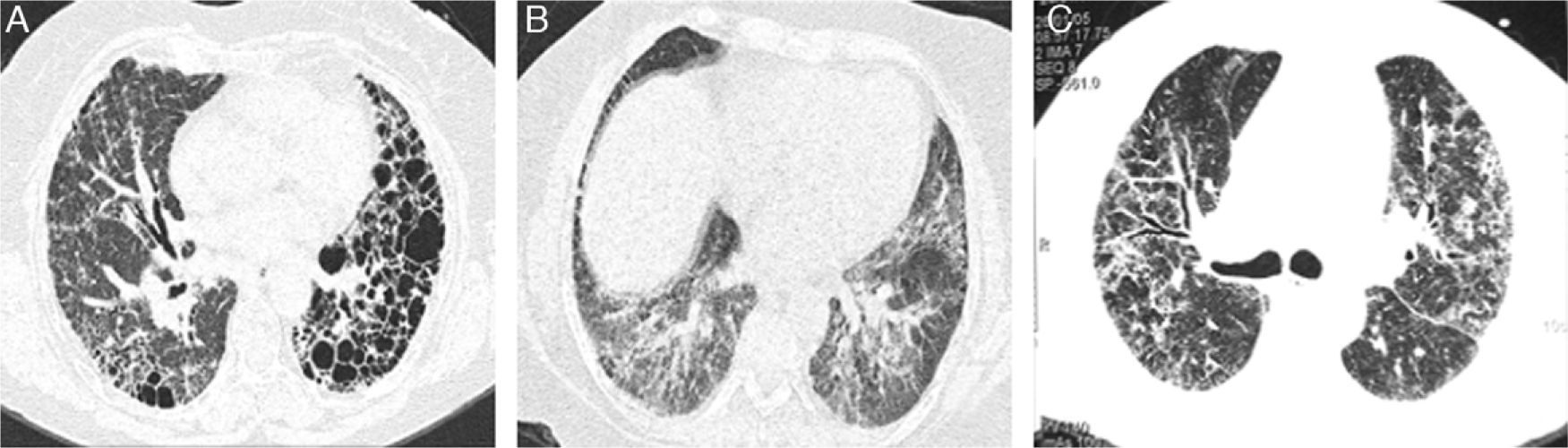
Representative CT images of RA-ILD patients. (A) Cystic lesions of subpleural and baseline distribution resembling those seen in usual interstitial pneumonia, associated with areas of ground glass. (B) Ground glass with central distribution, respecting subpleural areas and discrete reticulation, resembling the pattern observed in the nonspecific interstitial pneumonia. (C) Reticulation and ground glass diffusely distributed, broncocentric, being an indeterminate pattern of interstitial lung disease.
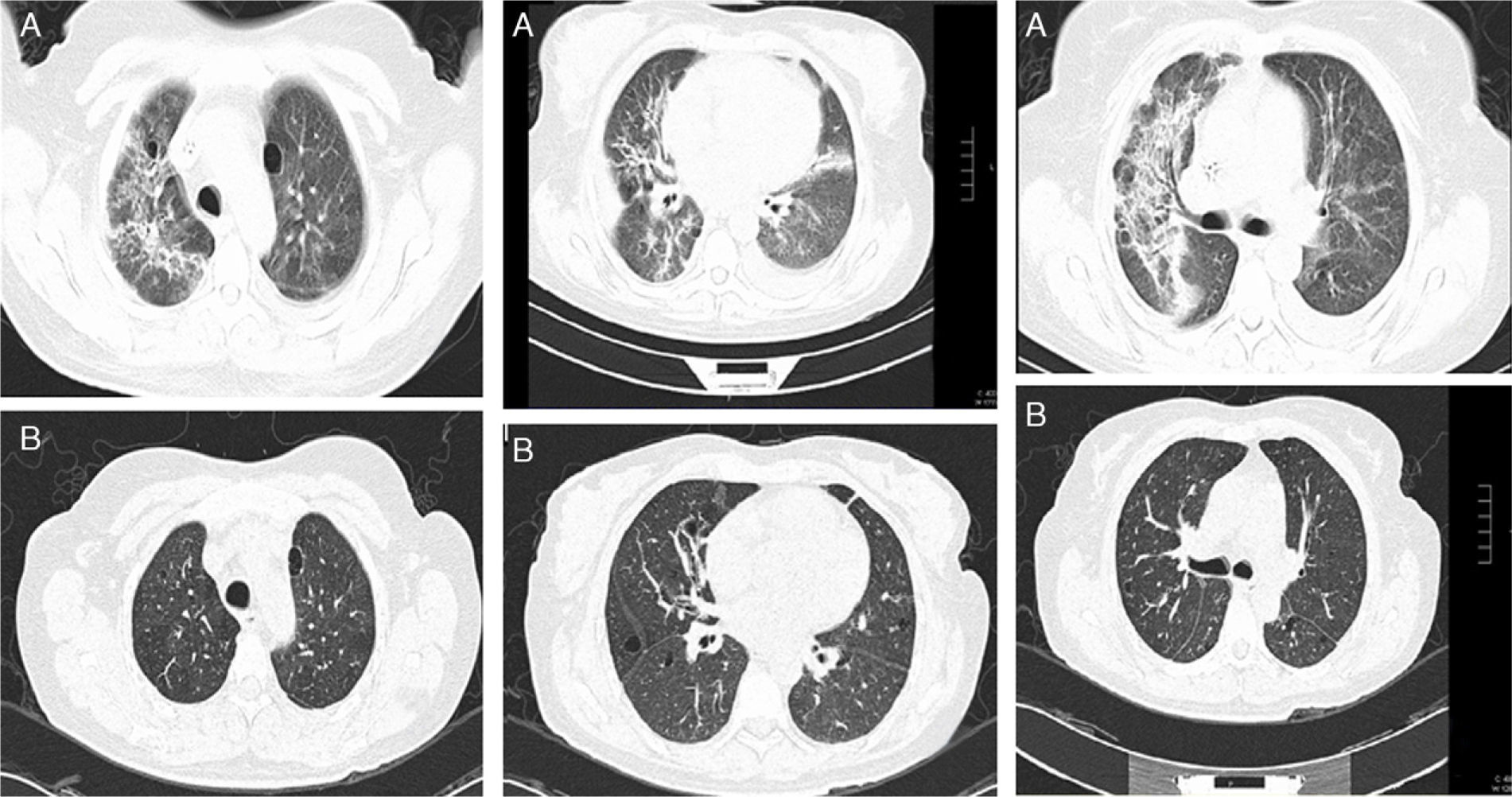
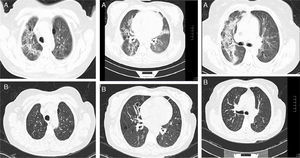
Baseline CT images of a patient with ILD related to RA. (A) Multiple cystic images with perivascular and random distribution, and diffuse ground glass opacities and patchy areas of lung consolidations with broncocentric interstitium thickening. (B) One year follow-up CT images after treatment with prednisone and methotrexate, showing less ground glass opacities, resolution of the consolidation areas and broncocentric interstitium thickening.
HRCT Findings According to The ATS/ERS Criteria and The Kazerooni Scale Score for Fibrosis and Ground Glass (Mean, IQR).
| HRCT Pattern, n=33 | Kazerooni Score (Mean, IQR) | |
| Ground Glass | Fibrosis | |
| UIP, n=4 | 1.49 (0.99–1.83) | 1.91 (1.41–2.33) |
| NSIP, n=12 | 2.49 (1.83–3) | 1.49 (0.99–1.83) |
| Inflammatory indeterminate, n17 | 2.5 (2–3) | 0 (0–0.33) |
ATS/ERS: American Thoracic Society/European Respiratory Society; HRCT: high resolution computed tomography; UIP: usual interstitial pneumonia; NSIP: nonspecific interstitial pneumonia.
Comparison of FVC Values at Baseline and at a Minimum of 6 Months of Follow-Up According to The Mean of The Kazerooni Fibrosis Scale Score of The Sample, Patients With Less of 0.47 Had a Statistically Significant Improvement of the FVC Baseline Values.
The results in this study show that patients with RA and ILD may have an improvement in the FVC after treatment with high doses of corticosteroids and DMARDs. This improvement is related to the degree of fibrosis present at the beginning of the medical treatment, because patients with lower Kazerooni fibrosis scores were the ones who showed improvement in the FVC values.
The general characteristics of the patients deserve some comment. First, although patients had a mean duration of RA symptoms of 64 months, only 40% of the patients had a history of previous use of DMARDs. So, the majority of EPI-RA patients described in this cohort had not been treated according to current guidelines. Although male sex has been described as a risk factor for EPI-AR, in our cohort we only had 4 male patients. The mean age of our patients, is very similar to what has been described in other cohorts.4
Nowadays, there is no consensus about the medical treatment in ILD related to RA. Although the prognosis in RA has improved in recent years and DMARDs, especially methotrexate has shown its efficacy and safety in the treatment of RA,11,12 the use of several DMARDs in the case of ILD related to RA is controversial. Some authors have suggested that methotrexate should not be used in a patient with ILD related to RA because of the risk of acute pneumonitis.13
Nevertheless, some recent data questions that recommendation. Park et al.14 have shown that RA patients with a usual ILD pattern are a high risk group for acute exacerbations defined by the Akira criteria.15 In this report, RA patients with a usual ILD pattern had a higher incidence of acute exacerbations compared to idiopathic pulmonary fibrosis patients. More importantly, no RA patient in this report was treated with methotrexate or other DMARDs previously described as a possible cause of acute pneumonitis. Kim et al.8 have reported that methotrexate and anti TNF-α treatment are not associated with worse prognosis in a cohort of ILD related to RA patients. Analyses of large data sets have not shown any evidence of association between rheumatoid arthritis treatment and the risk of severe interstitial lung disease.16 In conclusion, the results of this study and that of previous studies suggest that the recommendation of not giving methotrexate or other DMARDs to ILD related to RA patients is not accurate.
Another interesting finding in this study is the frequency of HRCT scans patterns. As Kim et al.8 reported, the most prevalent HRCT pattern was an indeterminate pattern with diffuse ground glass and reticulation in 50% of those patients with a baseline HRCT scan, followed by a NSIP in 38% and a usual pattern in 12% of the patients. The usual pattern is associated with a worse prognosis. We found no difference in survival according to the type of pattern, but our sample size and time of follow-up do not allow having firm conclusions in this regard. Nevertheless, patients with less than 0.47 fibrosis Kazerooni score had a better improvement of the FVC baseline values. The cut of value was selected arbitrarily because it was the mean of the Kazerooni scale for fibrosis in our patients.
There are several limitations in the present study, first its retrospective nature and that the treatments that patients received were selected by the treating physicians. This fact does not allow having a valid comparison of different DMARDs used in this cohort, and also, no conclusions about the long term prognosis should be made. Another limitation is the short follow-up of the patients, so we cannot estimate even if the improvement in FVC is sustained in time. We believe that clinical trials must be done to answer which DMARD is the best option to treat patients with ILD related to RA. These clinical trials ideally should compare the effect of traditional DMARDs and new biological therapeutic agents. Other important limitation is that no measurement of RA disease activity was taken.
In conclusion, patients with RA and ILD may have an improvement in the FVC after a treatment with high doses of corticosteroids and DMARDs. The improvement is seen in the patients with lower Kazerooni fibrosis scores.
Conflict of InterestThe authors declare no conflicts of interest.
Please, cite this article as: Rojas-Serrano J, et al. Interstitial Lung Disease Related to Rheumatoid Arthritis: Evolution After Treatment. Reumatol Clin. 2012;8(2):68–71.