The anserine syndrome is a common cause of knee pain. Infiltration with glucocorticoids has been evaluated in studies with low level of evidence and there are no published clinical trials to determine its usefulness. The objective of this study was to determine the efficacy and safety of the infiltration of methylprednisolone in the treatment of anserin syndrome.
MethodsWe conducted a clinical trial in 58 adult patients with anserin syndrome, which presented intra-articular pathology ruled that reflected pain in the medial aspect of the knee. The WOMAC scale was assessed at baseline and patients were randomized to receive an infiltration of lidocaine plus 40mg methylprednisolone acetate (group 1) versus xylocaine plus distilled water (group 2). Both groups received 100mg of diclofenac sodium for 10 days. The WOMAC scale was applied at 4 weeks and adverse events were recorded.
ResultsEquivalence was demonstrated in both groups for demographic variables and initial clinical evaluation. There was no statistical difference in the 3 domains of assessment of the baseline WOMAC score. The median baseline WOMAC in group 1 was 32 and in group 2 was 25.5 points. At 4 weeks it was 8 and 6.5 points, which corresponded to an improvement of 61.6% and 62.8%, respectively.
ConclusionThe infiltration with methylprednisolone in anserin syndrome is not superior to placebo in patients taking diclofenac measured by the WOMAC scale at 4 weeks. The incidence of adverse events did not show any differences either.
El síndrome anserino es una causa frecuente de dolor de rodilla. La infiltración con glucocorticoides ha sido evaluada en estudios con bajo nivel de evidencia y no se han publicado ensayos clínicos para determinar su utilidad. El objetivo del estudio es determinar la eficacia y la seguridad de la infiltración de metilprednisolona para el tratamiento del síndrome anserino.
MétodosEfectuamos un ensayo clínico en 58 pacientes adultos con síndrome anserino, a los que se les descartó patología intraarticular que reflejara dolor en la cara medial de la rodilla. Se evaluó la escala WOMAC basal y se aleatorizaron a recibir una infiltración de xilocaína más 40mg de acetato de metilprednisolona (grupo 1) versus xilocaína más agua destilada (grupo 2). Ambos grupos recibieron 100mg de diclofenaco sódico durante 10 días. Se realizó la escala WOMAC a las 4 semanas y el registro de eventos adversos.
ResultadosSe demostró equivalencia en ambos grupos para las variables demográficas y en la evaluación clínica inicial. No hubo diferencias estadísticas en los tres dominios de evaluación de la escala WOMAC basal. La mediana del WOMAC basal en el grupo 1 fue de 32 y en el grupo 2 de 25,5 puntos. A las 4 semanas fue de 8 y 6,5 puntos, que correspondió a una mejoría del 61,6 y 62,8%, respectivamente.
ConclusiónLa infiltración con metilprednisolona en el síndrome anserino no es superior al placebo en pacientes que toman diclofenaco medidos por la escala WOMAC a las 4 semanas. La incidencia de eventos adversos tampoco difirió.
Anserine syndrome (HS), also known as anserine bursitis or tendinobursitis, is a common cause of medial knee pain. There is controversy about the structure affected by the AS. Prevalence in Mexico is 0.34% (95% CI, 0.24–0.45), with foot pain and including regional pain conditions in the lower limb, as reported in a cross-sectional multistage, stratified, randomized study of 3 regions of the country with the methodology used in the COPCORD (Community Oriented Program in the Rheumatic Diseases) program.1
Given the lack of evidence of the structure involved in this syndrome and its easy clinical diagnosis, the latter is established on the basis of medial knee pain with inferomedial tenderness. No imaging studies are required. The key point lies in pain on the medial aspect of the tibia, 3–5cm distal to the knee joint line. There are no validated criteria for this disorder and the diagnostic utility of the maneuvers described above has not been evaluated.2
Its treatment is based on physiotherapy, oral NSAIDs and correction of predisposing factors (e.g. overweight). In the event that no response is seen then glucocorticoid3 infiltration is merited. In this latter form of treatment, there are no published clinical trials and the methodology4 lacks rigor.5
The aim of this study was to determine the efficacy and safety of the infiltration of methylprednisolone for the treatment of AS and the incidence of adverse events.
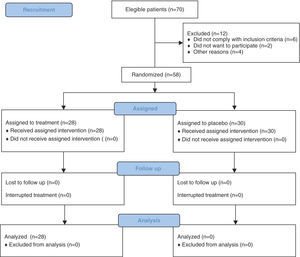
Materials and MethodsWe performed a randomized, double-blind, placebo-controlled trial at the rheumatology clinic specialty of the Internal Medicine Department of the Hospital Universitario Dr. José Eleuterio González. Fig. 1 shows the flowchart for recruitment, including monitoring and analysis of the participants. Patients were recruited from both sexes, 18–90 years of age with a prevalent clinical diagnosis of AS. We excluded patients who were carriers of intraarticular pathology reflecting the medial pain in the knee, such as meniscal disease, collateral ligament injury, medial plicature or in addition to glucocorticoids, were allergic to diclofenac or methylprednisolone, or had a coagulopathy that prevented the completion of the procedure. We also excluded, at the discretion of the investigator, patients with uncontrolled disease, including the presence of local or systemic infection. Also removed were patients who failed to comply with monitoring, used some unspecified treatment during the study and those who experienced adverse effects.
The study was previously approved by the ethics committee deputy director of research at the Faculty of Medicine and Hospital Universitario Dr. José Eleuterio González of the Universidad Autonoma de Nuevo Leon and registered in ClinicalTrials.gov. All patients gave written informed consent according to the General Health Law of Mexico.
To establish the diagnosis of SA, the principal investigator (VM) first asked the patient: do you have pain when climbing stairs? Is the pain on the medial aspect of the knee? If the answer was yes, palpation was performed at the affected site and the diagnosis established. During the physical examination maneuvers were performed to rule out intra-articular and periarticular pathology manifested with pain on the medial knee (meniscus, collateral ligaments, etc.). After obtaining informed consent, Western Ontario and McMaster Universities Arthritis Index (WOMAC) was filled.6 Subsequently, based on a random number table 2 generator,7 groups were assigned; group 1 received a single injection of methylprednisolone 40mg/1ml+2ml of 2% simple lidocaine and group 2 received a single injection of 1ml of distilled water+2ml of 2% simple lidocaine at the site of greatest pain. Both groups received 100mg of diclofenac sodium orally every 24h for 10 days. The injection technique was performed in the most painful spot4,8,9 using an aseptic technique and blinded (syringes covered) by a certified rheumatologist (NL) different from the evaluator. Participants were seen at 4 weeks to fill a new WOMAC questionnaire, visual analogue scale (VAS) satisfaction of the procedure and adverse event reporting. Complete remission was defined as a WOMAC score equal to 0 at week 4, in addition to quantifying the percentage of improvement from the first measurement.
The number of patients enrolled due to sample size calculation for a difference of proportions, where the proportion of patients was based on the complete response to infiltration with methylprednisolone (P1) versus the proportion of patients with complete response to the infiltration of placebo (P2), were compared. P1: 30%, P2: 5% with an alpha of 0.05 and a statistically significant bilateral 95% CI. We determined a beta of 0.20 for a power of 80%, obtaining a sample of 27 participants per group. A P<.05 was statistically significant.
We conducted a descriptive statistical analysis of the clinical and epidemiological numerical variables to determine their distribution and to determine their comparative parametric (t-test) or nonparametric analysis (Mann–Whitney). For categorical variables we used the chi-square test and Fisher exact test if required. We evaluated the randomization of participants with statistical tests of difference according to a variable distribution. WOMAC indices were compared at baseline and 4 weeks with the Wilcoxon rank test. To set the primary efficacy objectives of safety and secondary infiltration tests we used the Mann–Whitney and Fisher exact test, respectively.
Results58 participants were recruited: 28 were infiltrated with lidocaine and methylprednisolone (group 1) and 30 with lidocaine and distilled water (group 2). Table 1 shows the clinical and epidemiological characteristics together with the demonstration of homogeneity of each variable by group. The average age was 54.3 and 54.4 years, respectively. Women were 75% and 80% of those included in both groups. The average BMI was 30.9 and 30.6kg/cm2. There was no difference between the occupations of the participants. In most cases the pain was located in the left knee and the median duration of disease was 52 and 50 weeks in each of the groups. The median initial WOMAC in group 1 was 32, while in group 2 it was 25.5, with no statistical difference.
Baseline Clinical Epidemiologic Characteristics.
| Group 1 (n=28) | Group 2 (n=30) | P | |
| Age, years | 53.4 (12.5) | 54.4 (10) | .75 |
| Women, % | 21 (75) | 24 (80) | .65 |
| Weight, kg | 76.6 (14.1) | 73.3 (11.2) | .32 |
| Height, cm | 158.5 (8.2) | 158.7 (9) | .94 |
| BMI, kg/cm2 | 30.9 (4.1) | 30.6 (4.5) | .80 |
| Occupation, % | .26b | ||
| Home | 18 (64.3) | 17 (56.7) | |
| Employee | 1 (3.6) | 4 (13.3) | |
| Commerce | 7 (25) | 9 (30) | |
| Retired | 2 (7.1) | 0 | |
| Anserine syndrome of the right knee, % | 10 (35.7) | 14 (46.7) | .39b |
| Progression in weeksa | 52 (72) | 50 (187) | .74c |
| WOMAC initiala | 32 (24.8) | 25.5 (30.5) | .40c |
| Pain | 7 (2.9) | 7.1 (3.07) | .89 |
| Stiffnessa | 3 (4.75) | 3 (6) | .78c |
| Function | 22.5 (20.5) | 16 (25.3) | .31 |
Values expressed as mean and standard deviation.
Statistical test: Student's t.
BMI, body mass index; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
At 4 weeks, the median WOMAC was 8 and 6.5 points, respectively. The complete remission rate was 21% for group 1 and 30% for group 2. Both groups improved by 62% and 63% (statistically significant compared with intragroup baseline measurement). The median VAS satisfaction score was 87.5 and 70, respectively. Tables 2 and 3 show the rest of the evaluated variables and frequency of adverse events, and neither showed a statistically significant difference.
Evaluation of Efficacy at 4 Weeks.
| Group 1 | Group 2 | P | |
| WOMAC final | 8 (17) | 6.5 (17) | .77 |
| Pain | 2.5 (3) | 2 (5.5) | .78 |
| Stiffness | 0 (1) | 0 (1) | .21 |
| Function | 4.5 (14) | 4 (17) | .74 |
| VAS satisfaction | 87.5 (50) | 70 (65) | .32 |
| Complete remission, % | 6 (21) | 9 (30) | .45c |
| Percentage of improvementa | 61.6 (37) | 62.8 (33) | .89b |
Values expressed as median and interquartile range.
VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
We report the first clinical trial that allows exploration of the efficacy and safety of injection of glucocorticoid in patients with AS. We demonstrate the effectiveness, comparable to placebo infiltration, of methylprednisolone in a group of patients receiving concomitant diclofenac. Improvement was found in 60% of cases and remission at 4 weeks in 30% or so.
Among the advantages of this study are as follows: placebo-controlled design, blinding of infiltration and the scales used in the evaluations, which provide greater methodological rigor. The effectiveness evaluation was not reduced to a VAS, as previous4 reports, but rather evaluated in 3 domains of AS involvement in the patient (pain, stiffness and function).
Although the use of the WOMAC tool is one of the strengths of the study, it could also be a weakness. This scale was designed for patients with knee osteoarthritis and may have little sensitivity to measure pain, stiffness and function associated with AS alone, as there are items that do not necessarily reflect stress on the structure under study (e.g. functionality standing or morning stiffness). Even so, the individual assessment of pain score as measured by WOMAC showed no difference in both groups.
When comparing our results with previous studies, we found that Calvo-Alen et al. reported the results of 44 patients with AS who were assigned to receive 500mg of naproxen sodium 2 times daily or infiltration with glucocorticoids. The main outcome was pain assessed using a verbal scale of intensity with a monthly follow-up. 58% of the naproxen group had a “significant improvement” and in 5% the condition was resolved. In patients with infiltration, 70% had “significant improvement” and the condition was resolved in 30% (P<.05).4 The resolution rate is similar to that found in our trial in both groups infiltrated.
Similarly, in a retrospective review of 29 patients with AS, clinical remission was observed in 11 of 12 patients who received infiltration, compared with 7 of 17 of those not receiving it.5
Finally, Yoon et al.9 evaluated with ultrasound 26 patients diagnosed with osteoarthritis of the knee and AS. 17 of them received infiltration with triamcinolone acetonide in the bursa. The therapeutic response was evaluated by VAS and WOMAC scales, patient global assessment and the investigator using the Likert scale. Only 2 patients (8.7%) showed the presence of anserine bursitis/tendinitis by ultrasound. The VAS, the pain index and WOMAC functional capacity showed a statistically significant improvement after infiltration. The overall assessment of the patients showed an excellent response in 2 cases, good in 6 and moderate in 1; in 8 cases there was no improvement and none worsened. The initial WOMAC cohort evaluated scored 47 points and after treatment, 37 points. Two patients who reported an excellent response had evidence by ultrasonography of bursitis. Although this study infiltrated a steroid different from ours and made the effort to document the lesion with ultrasound, the procedure was not controlled and there was a satisfaction level similar to that presented by our patients in both groups.
Regarding demographics, the average age of participants was 63 years and BMI of 26kg/m2. They presented an average duration of illness of 93 months. By contrast, the present study population included had an average age 10 years lower, most were obese and the median duration of symptoms was 12 months.
In our series, we did not conduct ultrasound confirmation of the lesion and, unlike the study by Yoon and Kim, we use as methylprednisolone and triamcinolone acetate, common in our setting for infiltration.
Another possible disadvantage is that the application of the results of this study in clinical practice would obey only to patients within the age range established and using oral NSAIDs, which were used in a compassionate and ethical manner for group 2. In patients in whom the use of diclofenac is not possible, infiltration with methylprednisolone is recommended. This clinical scenario was not addressed in our work.
Diclofenac treatment was opted for, as it is an effective and safe anti-inflammatory for most of the participants and allowed us to control the use of other drugs by the patient, which was reflected in better control of the conditions of the study. We do not consider that its use modified the inflammatory response observed in the study, but maybe the magnitude of improvement, without changing the resulting difference.
When the results of a comparative test like this do not show the difference between the output variables, we must perform a test of statistical power. In our study this was between 90% and 95% and allowed us to establish with more certainty the results obtained in the trial. Still, we should consider that perhaps the sample size was poor and did not allow us to find a difference in the interventions, which was calculated based on the differences found in a blinded clinical trial 4. A future design could be considered with the percentage difference obtained in this trial and would probably find the clinical level expected by the active treatment.
As has been suggested in previous reports using a full placebo group, the infiltration of distilled water without Xylocaine corresponds to subtract the effect of lidocaine in the evaluation and thus has a cleaner measurement of the effect of glucocorticoid. However, we consider it unethical. Moreover, it should be pointed out that the absorption of lidocaine is 30min10 and it is unlikely that this causes long-term effect. However, we recognize that the effect of the use of lidocaine could be decisive for the 63% improvement of intra-group in group 2, but not enough to show differences between comparison groups. In a subsequent trial it would be appropriate to evaluate a single infiltration of lidocaine against placebo injection of distilled water.
It also suggests that the use of imaging techniques, mainly ultrasound and magnetic resonance imaging helps locate the affected structure in AS and helps infiltrating. This would also rule out, objectively, any intraarticular pathology, which would further reduce the bias in the evaluation of pain.11 However, the prevalence of AS in studies that seek intentionally is not very illustrative, and in addition to that, studies of knee injuries describe alterations in the bursa which are most prevalent and are even present in asymptomatic knees, so that the role of imaging is not yet defined.12,13
Similarly, it is important to track the long term to define the recurrence rate of AS group and thus investigate whether there is any superiority in using methylprednisolone.
Even taking into account the above considerations, we conclude that the use of an infiltration of methylprednisolone fails, compared to placebo, to achieve improvement at 4 weeks in patients with AS taking diclofenac.
Conflict of InterestThe authors have no conflict of interest to declare.
Please cite this article as: Vega-Morales D, et al. Eficacia y seguridad de la infiltración con metilprednisolona en pacientes con síndrome anserino: ensayo clínico aleatorizado. Reumatol Clin. 2012;8(2):63–7.