To analyze the changes in health-related quality of life (HRQoL) of patients with rheumatoid arthritis (RA) treated with biological therapies.
MethodObservational prospective study performed from October 2006 to May 2011. The inclusion criteria were adult patients, diagnosed with RA, treated for at least one year with anti-tumor necrosis factor therapy (infliximab or etanercept), who had not received other biological treatments previously. A total of 41 patients who completed the study undertook the specific and validated questionnaire QoL-RA Scale 3 times: E1 (September 2006–February 2007), E2 (April 2008–January 2009) and E3 (July 2010–May 2011). Data analysis was conducted using Epi-Info version 3.3 2004 for Windows® and Excel 2007; mean comparisons were evaluated by Student's t-test and the relationship between the 3 outcomes for each patient by lineal regression.
ResultsOverall results show a downward trend which was not statistically significant: 7.09 (standard deviation [SD]=1.15) in E1; 6.90 (SD=1.60) in E2; and 6.52 (SD=1.59) in E3. Items with higher scores were those related to psychosocial aspects (help from family, interaction with family and friends), whereas the physical dimension was valued more poorly (physical ability, arthritis pain, arthritis). Between E2 and E3 there was a significant increase in help from family (P=.0008), whereas level of tension (P=.0119) and mood (P=.0451) decreased significantly.
ConclusionsIn all, HRQoL reported by patients is good and has remained unchanged after approximately 6 years of study. The stability of HRQoL is probably partly attributable to treatment.
Analizar la evolución a largo plazo de la calidad de vida relacionada con la salud (CVRS) en pacientes con artritis reumatoide (AR) tratados con terapias biológicas.
MétodoEstudio observacional prospectivo realizado entre octubre de 2006 y mayo de 2011. Se seleccionó a pacientes mayores de edad, diagnosticados de AR, tratados durante al menos un año con anti-TNF (infliximab o etanercept) y que no hubieran recibido otros tratamientos biológicos previos. Completaron el estudio 41 pacientes, cumplimentando el cuestionario específico validado QOL-AR en 3ocasiones: E1 (entre septiembre de 2006 y febrero de 2007), E2 (entre abril de 2008 y enero de 2009) y E3 (entre julio de 2010 y mayo de 2011). Los datos se analizaron con Epi-Info versión 3.3-2004 para Windows ® y Excel 2007; la comparación de las medias con la prueba t de Student y la relación entre valores de un mismo paciente, mediante regresión lineal.
ResultadosResultados globales: tendencia descendente no estadísticamente significativa: 7,09±1,15 en E1; 6,90±1,60 en E2 y 6,52±1,59 en E3. Los ítems con mayor puntuación fueron los relacionados con aspectos psicosociales (ayuda familiar, interacción con familia y amigos). La dimensión física fue la peor valorada (habilidad física, dolor artrítico, artritis). Entre E2 y E3 aumentó significativamente la valoración de la ayuda familiar (p=0,0008) y disminuyeron significativamente tensión nerviosa (p=0,0119) y estado de ánimo (p=0,0451).
ConclusionesLa CVRS de los pacientes es buena y se ha mantenido prácticamente invariable tras unos 6años de estudio. Es probable que parte de la estabilidad en la CVRS sea atribuible al tratamiento.
Rheumatoid arthritis (RA) is a chronic autoimmune disease of unknown etiology, that can affect people of any age and sex, although its incidence is greater in women (11.3 per 100000 population; 95% confidence interval [CI]: 10.0–12.8) than in men (5.2 per 100000 population; 95% CI: 4.3–6.3).1 The prevalence of RA in the adult Spanish population is 0.5%–0.8%; more than 200000 patients are affected and 20000 new cases are diagnosed each year.2
The symptoms of this disease (pain, fatigue, deformity and loss of joint movement) alter the life of those affected, reducing their capacity to engage in activities of daily living and work, altering their mood and, in short, reducing their quality of life, both overall, and in each of the physical and psychosocial dimensions involved. This impact has been confirmed by a number of indicators and questionnaires.3–6 The effect of RA on health is more negative than that of other diseases like hypertension, diabetes or acute myocardial infarction and, with the exception of the mental component, even more than depression.7,8
Biological therapies have substantially modified the course of the disease; although they do not result in complete remission, they have been found to be efficient in reducing the inflammatory activity, detaining its progression and preventing joint damage. Therefore, biological drugs constitute an interesting therapeutic alternative in that they prevent disability and maintain the autonomy of RA patients for as long as possible and, thus, their physical, mental and social well-being.9
The health-related quality of life (HRQoL) is a subjective and multidimensional variable that is fundamental in the management of chronic diseases like RA, as it enables the determination of how patients perceive their health status, the impact of the disease and response to treatment. At the present time, it is a necessary complement to the classical indicators, making it possible to undertake an in-depth estimation of the effectiveness and efficiency of health programs or interventions and, consequently, facilitate decision making in this respect.10
The purpose of our study was to provide information on an aspect that has not been dealt with to date: the long-term changes in HRQoL in RA patients treated with biological therapies, applying a specific questionnaire.
Materials and MethodsWe conducted an observational prospective longitudinal study between October 2006 and May 2011. This single-center study was performed in a 700-bed general hospital that attended a population of 350000 inhabitants. We selected patients who were 18 years of age or older and had been diagnosed with RA. They had been treated with the biological agents that were available when the study was initiated (infliximab or etanercept) for at least 1 year and had not received other biological therapies. The sociodemographic characteristics were obtained from the medical records.
Criteria for exclusion from the study were voluntary withdrawal, discontinuation of the biological therapy for any cause and the development of comorbidities that could interfere with responses to the questionnaire.
To determine their HRQoL, we employed the specific and validated Quality of Life in Rheumatoid Arthritis Scale (QOL-RA)11 given that the results have a high level of correlation with those of the Health Assessment Questionnaire (HAQ), which assesses functional capacity in patients in terms of their performance of activities of daily living, with the Disease Activity Score in 28 joints (DAS28) to determine disease activity, Visual Analog Scale for pain and the modified Sharp score for radiological progression.12,13 The QOL-RA scale consists of 8 items with 10possible responses, which range from 1 (very poor quality of life) to 10 (excellent quality of life) and evaluates both the physical as well as the psychological and social dimensions. It provides a global assessment obtained from the mean of the values of each item. It was validated both in English and Spanish in Los Angeles, California, United States. The Spanish version incorporates Mexican, Central American and South American linguistic idiosyncrasies. The internal consistence of the Spanish version has a Cronbach's alpha of 0.87.
The questionnaires were administered to the patients by members of the staff trained in surveys on quality of life and, specifically, in the QOL-RA scale. The patients received information on the objectives and characteristics of the study and gave their consent to participate. The study was approved by a clinical research ethics committee. During the study period, the questionnaire was administered on 3 occasions, the first between September 2006 and February 2006 (E1), the second between April 2008 and January 2009 (E2) and the third between July 2010 and May 2011 (E3).
The analysis of the data was carried out using the software package Epi-Info version 3.3 2004 for Windows and Excel 2007. The comparison of the means was performed using Student's test. A P value <.05 indicated statistical significance.
ResultsTable 1 shows the demographic characteristics of the patients who initiated the study and of those who completed follow-up. There were no significant differences between the 2 groups. In both there was a predominance of women who were approximately 61 years old and had devoted their time to housework.
Sociodemographic Characteristics of the Patients.
| Patients who initiated the study | Patients who completed it | |
|---|---|---|
| No. of patients | 81 | 41 |
| Sex (%) | 21 men (25.93) | 8 men (19.5) |
| 60 women (74.07) | 33 women (80.5) | |
| Paid work (%) | No: 27 (33.3) | No: 13 (31.7) |
| Yes: 54 (66.6) | Yes: 28 (68.3) | |
| Disease duration in years | 10.99±8.05 | 10.65±7.95 |
| Years being treated with anti-TNF-α | 2.40±1.30 | 2.52±1.23 |
| Age (years) | 54.46±15.81 | 53.66±13.32 |
Anti-TNF-α, anti-tumor necrosis factor alpha.
Statistically insignificant differences: P>.05.
Initially a total of 81 patients (58 women and 23 men) participated in the study. In all, 42 were being treated with infliximab and 39 with etanercept. The study was completed by 41: 21 with infliximab, 16 with etanercept and 4 with new biological agents (1 with adalimumab, 1 with abatacept and 2 with tocilizumab). Throughout the study, global treatment switches were carried out in a total of 7 patients.
The mean age of the patients when they initiated treatment with anti-tumor necrosis factor (anti-TNF) was 53.66±13.32, after a disease duration of 10.65±7.95 years. All of them had a DAS28>3.2 when they began treatment. The time elapsed between initiation of treatment and the end of E1, E2 and E3 was 2.52±1.23, 4.20±1.37 and 6.39±1.34 years, respectively.
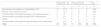
Table 2 summarizes the overall scores in the surveys and broken down according to item. Among the aspects regarded most highly by the patients in the 3 surveys were those that referred to family support, to interaction with family and friends and to mood; in contrast, those related to the physical dimension such as physical ability and arthritis pain were viewed the least favorably. There were statistically significant differences upon comparing E2 and E3 in psychosocial aspects such as family support (P=.0008), stress (P=.0119) and mood (P=.0451).
Values (Mean and Standard Deviation) of the Overall Results and Broken Down by Item in the Quality of Life in Rheumatoid Arthritis Scale in E1, E2 and E3, and P Value Upon Comparing E1 and E2 and E2 and E3.
| Item | E1 mean±SD | P (E1 vs E2) | E2 mean±SD | P (E2 vs E3) | E3 mean±SD |
|---|---|---|---|---|---|
| Physical ability | 6.51±1.61 | .3620 | 6.23±1.98 | .2399 | 5.98±2.06 |
| Support | 8.78±1.71 | .8740 | 8.49±1.89 | .0008 | 8.56±1.99 |
| Arthritis pain | 6.17±1.77 | .8197 | 5.80±2.52 | .2201 | 5.46±2.29 |
| Stress | 6.56±2.04 | .1386 | 6.59±2.33 | .0119 | 6.15±2.52 |
| Health | 6.51±1.75 | .1468 | 6.46±2.17 | .3229 | 5.83±2.19 |
| Arthritis | 6.41±1.75 | .3267 | 6.10±2.21 | .7807 | 5.63±2.17 |
| Social life | 8.58±1.91 | .6942 | 8.29±1.90 | .0781 | 7.88±2.16 |
| Mood | 7.24±1.83 | .4788 | 7.12±1.99 | .0451 | 6.68±2.56 |
| Total | 7.09±1.15 | .0629 | 6.90±1.60 | .4061 | 6.52±1.59 |
SD, standard deviation.
The overall difference between E2-E1 and E3-E2 shows a nonsignificant deterioration of 0.19±1.68 and 0.38±1.79 points, respectively.
The dropouts that occurred during the study are shown in Table 3. In all, 17 of these cases (42.5%) were due to the development of symptoms attributable to other causes rather than to RA, that could have an impact on the responses to the specific questionnaire. The development throughout the study of psoriasis, spondyloarthropathies, inflammatory bowel disease, depression or schizophrenia and the need for surgical interventions, among others, resulted in the exclusion of these 17 patients from the analysis.
Dropouts Throughout the Study and Causes.
| Dropouts E1–E2 | Dropouts E2–E3 | Total | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Development of symptoms not attributable to RA | 15 | 37.5 | 2 | 5 | 17 | 42.5 |
| Refusal, lack of access or errors | 5 | 12.5 | 4 | 10 | 9 | 22.5 |
| Discontinuation of biological therapy due to adverse reactions | 4 | 10 | 2 | 5 | 6 | 15 |
| Discontinuation of biological therapy due to refractoriness | 1 | 2.5 | 2 | 5 | 3 | 7.5 |
| Others | 3 | 7.5 | 0 | 0 | 3 | 7.5 |
| Discontinuation of biological therapy because it was unnecessary | 1 | 2.5 | 1 | 2.5 | 2 | 5 |
| Total | 29 | 72.5 | 11 | 27.5 | 40 | 100 |
RA, rheumatoid arthritis.
A total of 9 cases (22.5%) were due to the refusal of the patient to respond, to the inability to contact the patient during the period established for the administration of the questionnaire, or to errors in the identification, processing or interpretation of the responses. The decision was made to discontinue treatment in 15% of the participants, since it was considered that the risks were greater than the beneficial effects owing to adverse effects such as infections, urticaria or heart failure; in 7.5%, biological therapy was considered ineffective in patients with refractory disease and in 5% because it was considered unnecessary in those with stable RA. The remaining 7.5% were excluded due to other causes such as lack of adherence or death.
The overall results of the surveys, without excluding patients who did not complete the study were: E1 (n=81), 6.78±1.61; E1 (n=52), 6.97±1.38; E1 (n=41), 7.09±1.15; E2 (n=52), 6.70±1.78; (n=41), 6.90±1.60. The differences between the values in E1 and the same values in E2 did not reach statistical significance.
DiscussionAlthough cross-sectional studies that analyze the short-term impact of a drug provide relevant information, they have a limited perspective for chronic diseases like RA, in which long-term studies are indispensable. For this reason we feel that the longitudinal design of our report proves to be of interest.
After the study had been initiated, the Spanish questionnaires that share objectives with the QOL-RA scale were validated, and possibly with better psychometric characteristics,14,15 although it did not lead to a change in the tool. The simultaneous application of these questionnaires could provide valuable information on their validity.
Changes in the HRQoL show a downward trend, although this decrease is not statistically significant. Overall, we observe a reduction in the HRQoL of 0.19 points at the first cutoff point and of 0.38 at the second. If we consider that the period elapsed between the observations is more and a year and a half, these values signify that we can speak of maintenance and stability in the HRQoL of the patients, rather than a reduction.
Taking into account that, in the study conducted by Ábalos et al.,9 in patients very similar to ours, the average value reported by the authors after application of the questionnaire prior to beginning treatment with infliximab or etanercept was 3.00±0.77, we can consider that our patients had a good HRQoL. Values of 7.09±1.15 in E1, 6.90±1.60 in E2 and 6.52±1.59 in E3, versus a possible maximum of 10, are satisfactory and coherent with respect to those of other similar studies,11,16–18 in which the same questionnaire was employed and the results of which are shown in Table 4. Both in the study performed in a population with characteristics similar to those of ours published by Fernández-Lisón et al.,16 and in the study by Danao et al.13 in populations of Anglo-Saxons and of Hispanics living in the United States, the results were somewhat lower, with means of 5.90, 5.54 and 5.28, respectively. However, in the studies of Vinaccia et al.17 and Prada et al.,18 performed in a Colombian and a Cuban population, respectively, with characteristics different from ours, the results obtained were slightly higher. This fact appears to indicate that cultural aspects can influence the perception of health and that, thus, must be taken into account.
Overall Results of the Health-related Quality of Life Questionnaire Broken Down by Item (Mean±Standard Deviation) in Cross-sectional Studies Utilizing the Quality of Life in Rheumatoid Arthritis Scale.
| Item | Fernández Lisón et al. | Danao et al. | Vinaccia et al. | Prada et al. | |
|---|---|---|---|---|---|
| Anglo-Saxons | Hispanics | ||||
| Physical ability | 5.42±1.67 | 5.76±1.98 | 5.29±1.89 | 7.74±1.92 | 6.91 |
| Support | 7.45±2.10 | 6.95±2.19 | 6.49±2.21 | 8±2.21 | 7.81 |
| Arthritis pain | 5.10±1.83 | 5.46±2.31 | 4.76±2.39 | 6.72±2.35 | 6.12 |
| Stress | 5.50±2.01 | 5.74±2.22 | 5.65±2.03 | 6.82±2.63 | 7.11 |
| Health | 5.50±1.77 | 5.6±2.17 | 5.84±2.26 | 7.06±2.22 | 6.32 |
| Arthritis | 5.15±1.86 | 5.28±2.28 | 4.99±2.04 | 6.83±2.42 | 6.92 |
| Social life | 7.08±1.96 | 7.21±2.32 | 6.49±2.21 | 8.16±1.89 | 7.72 |
| Mood | 6.02±2.03 | 6.22±2.19 | 6.08±2.14 | 7.41±2.19 | 7.32 |
| Total | 5.9 | 5.54 | 5.28 | 7.32 | 7.03 |
The stability we allude to with respect to the mean of the quality of life is also observed independently in each item. We can point out a significant increase between E3 and E2 in the item on family support, in contrast to the rest of the results; there is a statistically significant decline between E3 and E2 in the items dealing with stress and mood. It may be interesting to point out that both items explore the psychological status of the patient. The duration of the disease could be an important element with respect to the perception of the patient, with regard to the tiredness resulting from deterioration. Data have shown how patients in some way adapt to their disease and that the limitations that it produces, in some way, favorably influence their perception of quality of life, although their capacities may be impaired. In our study, it seems that this adaptation is not clearly produced in questions of mood, although it may occur in the physical and social dimensions.
Coinciding with the results of Fernández-Lisón et al.,16 Danao et al.,11 Vinaccia et al.17 and Prada et al.19 and, despite the differences between the populations studied, the items with the highest scores are related to the psychosocial dimension (support from family and interaction with family and friends) in which the behavior of others is regarded more than that of the patient, whereas the physical dimension is valued the least (physical ability, arthritis pain, arthritis), as can be expected, given the characteristics of the disease.
With respect to the sociodemographic analysis of our patient population, the predominant profile was that of a woman of approximately 61 years of age who had devoted her time to housework. On the other hand, in E1 and in E2, half of the patients were receiving treatment with etanercept and the other half with infliximab, the 2 drugs most widely utilized for this indication, especially during the periods being studied. In E3, 4 patients received second-line biological agents. This reflects the course of the disease, in which, for different reasons, the therapeutic management has to be changed. The time elapsed since the initiation of biological therapy (6.39 years) means that these changes can be expected.
The periods of time elapsed between the surveys (1.68 years between E1 and E2, and 2.19 years between E2 and E3) do not respond to a specific strategy. As we have no references as to the length of time that would be adequate to observe variations in the quality of life of these patients, the second cutoff point after approximately a year and a half had an exploratory intention. The outcome observed with this first approximation indicated great stability in the results. Thus, shorter periods did not seem to be suitable, and our third cutoff point occurred more than 2 years later. However, the results again confirm marked stability and, therefore, the difference in periods between surveys does not appear to be important.
The study was proposed as an exploratory experience, in which the methodology was adapted to the circumstances of real-world clinical practice, assessed in previous studies.19 Therefore, we did not evaluate the impact of the treatment on naïve patients, the object of another report, nor did we go beyond the nearly 4 years of follow-up. In this respect, our article indicates what, how and when to collect data in future research—which is absolutely necessary—that includes more complex designs, with comparative groups, among other contributions.
The large number of patients who dropped out of the study is surprising; nearly 50% of the participants initially recruited did not complete follow-up. The objective of this report was to analyze how HRQoL changes in patients treated with biological therapies and, thus, the discontinuation of the drug, for any reason, was considered to be an exclusion. Nevertheless, the greatest number of dropouts was due to the development of symptoms that were not attributable to RA, but to other comorbidities. In a meta-analysis to assess the impact of RA on the HRQoL, randomized controlled trials were excluded for this purpose, due to the low levels of comorbidity that are usually observed in the recruited patients.8 The analysis of the sociodemographic characteristics of the population we recruited at the beginning and of those who completed the study, after nearly 6 years, showed no statistically significant differences. On the other hand, significant differences were not found on comparing the results of the questionnaires corresponding to E1 and E2, in the recruited population and the group that completed the study. Nevertheless, our results can only be applied in populations that meet the requirements called for in our patients: maintenance of the biological therapy and the lack of symptoms not attributable to RA, which in health practice is a limitation.
It would have been interesting to study the relationship of our results on quality of life with other variables, particularly involving clinical content. Scores and questionnaires like the HAQ assessing functional capacity, the Clinical Disease Activity Index and the DAS28 for clinical activity, are routinely utilized in the evaluation of the disease. This would possibly lend greater strength to our results. However, studying them went beyond the objectives of our report and would have required a design and resources that were not within our means, especially having planned a long-term study. On the other hand, the literature endorses a coherent relationship between the quality of life (with this and other questionnaires) and clinical assessment.18,20
The economic implications of a chronic disease like RA, as well as that of the treatments, are objectives worthy of concern and study.20,21 The search for tools that enable us to evaluate the real utility of biological therapies, especially that perceived by the patient, is a challenge that is underway, although it can be controversial.22,23 We had no data to indicate the changes in the HRQoL over the course of time in patients with a sociodemographic and clinical situation similar to ours, before the introduction of biological therapy. Despite the absence of these data, it is feasible to assume that the drugs utilized may have contributed to the maintenance of the quality of life in the patients we studied.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Authorship/collaboratorsAll of the authors participated in the design of the report, recruitment, data processing and the writing of the article.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Ortega-Valín L, Mayorga-Bajo I, Prieto-Fernández C, del Pozo-Ruiz J, Gutiérrez-Gutiérrez E, Pérez-Sandoval T. Evolución a largo plazo de la calidad de vida en pacientes con artritis reumatoide tratados con terapias biológicas. Reumatol Clin. 2018;14:191–195.