To compare the survival of subcutaneous anti-tumor necrosis factor (TNF) drugs used between 2008 and 2012 prescribed in accordance with clinical practice.
Material and methodsRetrospective, observational study of the patients in our center diagnosed with rheumatoid arthritis (RA). We included patients who had received a subcutaneous anti-TNF agent for at least 6 months. The data were analyzed using the SPSS V17.0 statistical package.
ResultsForty-nine RA patients started subcutaneous biological treatment with an anti-TNF agent (32 with etanercept and 17 with adalimumab). The mean age was 45.94 years (75.5% female). The mean disease duration prior to starting anti-TNF administration was 2.67 years. The mean age at the start of treatment was 51.84 years, and the average Disease Activity Score 28 was 4.93. The median survival of the anti-TNF treatment was 8.40 years; the survival of etanercept was the longer of the two. The main reason for discontinuation was secondary failure (90.9%).
ConclusionsIn routine clinical practice, the survival of subcutaneous anti-TNF treatment was extensive and was independent of whether or not the patients received concomitant immunosuppressive therapy.
Comparar la supervivencia de los anti-TNF subcutáneos utilizados durante el periodo 2008-2012 según práctica clínica.
Material y métodosEstudio observacional retrospectivo de todos los pacientes diagnosticados de AR que habían iniciado tratamiento con un anti-TNF subcutáneo y mantenido durante al menos 6 meses. Los datos fueron analizados mediante SPSS V17,0.
ResultadosCuarenta y nueve pacientes con AR iniciaron tratamiento con anti-TNF subcutáneo (32 con etanercept y 17 con adalimumab). La media de edad fue de 45,94 años (75,5% mujeres). La media de duración de la enfermedad previa al inicio del anti-TNF fue de 2,67 años. La media de edad al inicio del tratamiento fue de 51,84 años, índice de actividad de la enfermedad en 28 articulaciones medio de 4,93. La supervivencia media del tratamiento anti-TNF fue de 8,40 años, mostrando una mayor supervivencia etanercept. La principal razón de discontinuación fue por fallo secundario (90,9%).
ConclusiónEn la práctica clínica habitual, la supervivencia a largo plazo de los tratamientos anti-TNF subcutáneos fue elevada e independiente de que tuvieran o no tratamiento inmunosupresor concomitante.
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by inflammation of the synovial membrane that can result in destruction of the cartilage and joint damage, and is a major cause of disability.1 The mainstay of treatment is the early introduction of disease-modifying drugs (DMARDs) to control inflammatory activity.2,3
Recent years have seen a significant change in the treatment of RA with the introduction of the biological DMARDs. Anti-tumor necrosis factor drugs (TNF) are used when synthetic DMARDs have not achieved control of the disease. Clinical trials with various anti-TNF drugs have shown improvement in clinical inflammatory activity, functionality, overall health status assessment, and in the prevention of radiographic progression.4,5
Survival studies are an indirect way of assessing the effectiveness and safety of a drug in daily clinical practice,6 unlike clinical trials that include a selected patient population and are of short duration. Various factors can influence drug survival, and the main reasons for discontinuing a drug can be adverse effects and loss of efficacy.7 Furthermore, each rheumatic disease and the different biological treatments have a different survival profile.8,9
The aim of our study was to choose a cohort of patients from northeast Spain, with well-defined features to assess and compare survival and the main causes of discontinuation of subcutaneous anti-TNF drugs over the period 2008–2012. These drugs were prescribed in our center by the Rheumatology Department according to routine clinical practice.
Material and MethodsThis was a retrospective observational study of all the patients with a previous diagnosis of RA, attended consecutively in the Lucus Augusti University Hospital between 2008 and 2012. The patients included in the study had been diagnosed by their usual rheumatologist, and met the ACR criteria of 1987.10 Previous treatment with at least one DMARD had failed for all the patients, and therefore they had started treatment with a subcutaneous anti-TNF drug following the recommendations of the SER consensus document. In order to be included in the study, every patient who had started treatment with a subcutaneous anti-TNF had to have been maintained for at least 6 months.
To that end, we created a database that included epidemiological variables (age, sex, race, profession, employment status and date of disease diagnosis, clinical data (disease activity score in 28 joints (DAS28), rheumatoid factor, erythrocyte sedimentation rate and C reactive protein), and treatment data (date that the biological treatment was started, concomitant DMARDs, concomitant mean dose of corticosteroids, and intake of non-steroidal anti-inflammatory drugs [NSAIDs]). The reasons for discontinuing the anti-TNF drug were analyzed and classified into 3 categories: inefficacy (no primary or secondary response), adverse effects and other (remission of the disease, wanting to become pregnant, and patient preference).
The study was approved by the Hospital's ethics committee. The data were obtained by revision of the clinical histories. Before inclusion in the study, all the patients gave their informed consent.
Statistical AnalysisWe performed a statistical analysis of all the variables. The qualitative variables were analyzed by absolute and relative frequencies, while the quantitative variables were represented using mean, standard deviation and confidence intervals, if they followed normal distribution, or using median, minimum, maximum and interquartile range if they did not.
The Student's t-test was used to find statistically significant differences in parametric quantitative variables, and the U Mann–Whitney test for the non-parametric variables. Pearson's chi-squared test or Fisher's exact test were used for the qualitative variables for tables (2×2), and the verisimilitude test for tables (m×n).
The Student's t-test for paired data or Wilcoxon's test was used to study the association of quantitative variables with paired data according to the distribution of the variable. The McNemar test was used for qualitative variables for tables (2×2).
The survival function using the Kaplan–Meier method was used to describe the survival of the anti-TNF treatment. We used the Log Rank test to detect statistically significant differences in the survival functions.
The estimates were made with a 95% confidence level. They were considered significant with a P value <.05.
The data were analyzed using SPSS V17.0.
ResultsA total of 49 patients with RA started biological treatment with subcutaneous anti-TNF drugs in the period 2008–2012.
The mean age of the population was 45.94 years (SD 14.61), 75.5% were female. The mean duration of the disease prior to starting anti-TNF treatment was 2.67 years (SD 6.97). Of the total number of patients, 39 (79.6%) were rheumatoid factor positive. Before starting anti-TNF treatment, all the patients had been treated with at least one DMARD, methotrexate was used in all cases (mean weekly dose of 19.43mg). Leflunomide (doses of 19.20mg/day) was the second most frequently prescribed DMARD (53.1%), in the event of failure of or intolerance to methotrexate. Forty-four patients (89.8%) were being treated with corticosteroids (mean dose 10mg/day) and 45 (91.8%) with NSAIDs.
Table 1 shows the clinical progression characteristics of these patients. The available subcutaneous anti-TNF drugs were etanercept and adalimumab. Of the total number of patients, 32 (65.3%) were given etanercept, and 17 (34.7%), adalimumab.
At the start of treatment with anti-TNF drugs, the patients studied had a mean age of 51–84 years (SD 14.67) with a mean DAS28 of 4.93 points (SD .70); 4.79 in the etanercept group (SD .60), and 5.19 in the adalimumab group (.82), and no statistically significant differences were observed between either subpopulation (P=.110).
Seventy-six percent of the patients who started treatment with biological treatment maintained DMARD treatment. In these cases, methotrexate was the most frequently combined drug (73%). The proportion of patients who maintained concomitant treatment with methotrexate was higher among those who started treatment with adalimumab (76.5% vs 43.8%, P=.038).
Once anti-TNF treatment had been started, the corticosteroids were discontinued for 16 patients (36.4%) (P<.001), 27% in the etanercept group, and 59% in the adalimumab group (P<.05).
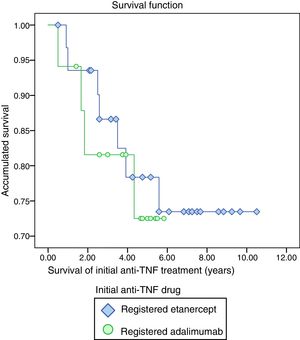
The mean survival of the initial anti-TNF treatment was 8.40 years (CI 7.32–9.48), etanercept showed the highest survival (8.53 years, CI 7.26–9.80) compared to adalimumab (4.87 CI 4.01–5.73), although there was no statistically significant difference (P=.706) (Fig. 1).
Anti-TNF treatment was discontinued definitively for 11 patients (22.4%). The main reason for discontinuation was secondary failure (90.9%). Only 9.1% was due to primary failure. The proportion of anti-TNF treatment discontinuation was similar in both the etanercept and the adalimumab group.
DiscussionIn our study, in daily clinical practice, the discontinuation rate of biological treatment at 5 years was 22.4%. This result shows greater survival than previous studies, which describe a discontinuation rate of 49.7% at 2 years.6
With regard to mean anti-TNF treatment survival, an indirect measure of the effectiveness of the drug, in our population etanercept showed higher survival than adalimumab. Our results are similar to those published by Neovius et al.11 and those found in an Italian study12 on clinical practice, where they compared response to the treatment and the rate of remission of patients treated with adalimumab, etanercept or infliximab, and concluded greater retention for etanercept compared to adalimumab or infliximab. Furthermore, a recent metaanalysis13 that included 98 studies showed similar results, but with a significantly higher retention rate for etanercept than for infliximab and adalimumab.
The main reason for definitive discontinuation in our study was loss of efficacy. These findings are consistent with the study by García-Lagunar et al.,14 where the treatment discontinuation rate due to inefficacy was greater than that due to secondary effects. Identifying antibodies to the drug might be particularly relevant with regard to loss of efficacy.15 For technical reasons, we did not have this information for our study.
Our study's main limitation was the small sample size. Therefore the differences found between the etanercept and adlimumab groups in terms of discontinuation rate did not achieve statistical significance. In the period over which we carried out our study only 2 subcutaneous anti-TNF drugs were available on the market.
In conclusion, in our center and in routine clinical practice, the long term survival of subcutaneous anti-TNF treatments was high, irrespective of whether or not there was concomitant immunosuppressant treatment. The main cause for discontinuing the treatment was due to secondary failure.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that neither human nor animal testing has been carried out under this research.
Data confidentialityThe authors declare that they have complied with their work center protocols for the publication of patient data.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is held by the corresponding author.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Alvarez Rivas N, Vazquez Rodriguez TR, Miranda Filloy JA, Garcia-Porrua C, Sanchez-Andrade Fernández A. Supervivencia a largo plazo de los fármacos biológicos anti-TNF subcutáneos administrados durante los años 2008-2012 en una cohorte de pacientes con artritis reumatoide. Reumatol Clin. 2019;15:54–57.