Reactive hemophagocytic lymphohistiocytosis (HLH), or macrophage activation syndrome (MAS), is an unusual complication of systemic inflammatory diseases.1 Its association with juvenile idiopathic arthritis (JIA) is rare and treatment must be immediate. We report the case of a girl in whom we detected this association and treated it successfully with a drug that is not first-line therapy because of its potential adverse effects.
The patient was a 9-year-old girl who had recently been diagnosed as having JIA. She was admitted to the hospital after a 3-day history of persistent fever (40°C) and generalized facial edema, presumably associated with methotrexate she was taking, a treatment that was interrupted. At admission, she was pale, had painful hepatosplenomegaly and an increase in the volume of several joints. Laboratory tests showed pancytopenia (hemoglobin 6.4g/dL; 132,000 platelets/mm3 and 1080 neutrophils/mm3), prothrombin time and partial thromboplastin time were increased (16.8″ and 59.2″, respectively) and fibrinogen was decreased (164.7mg/dL).
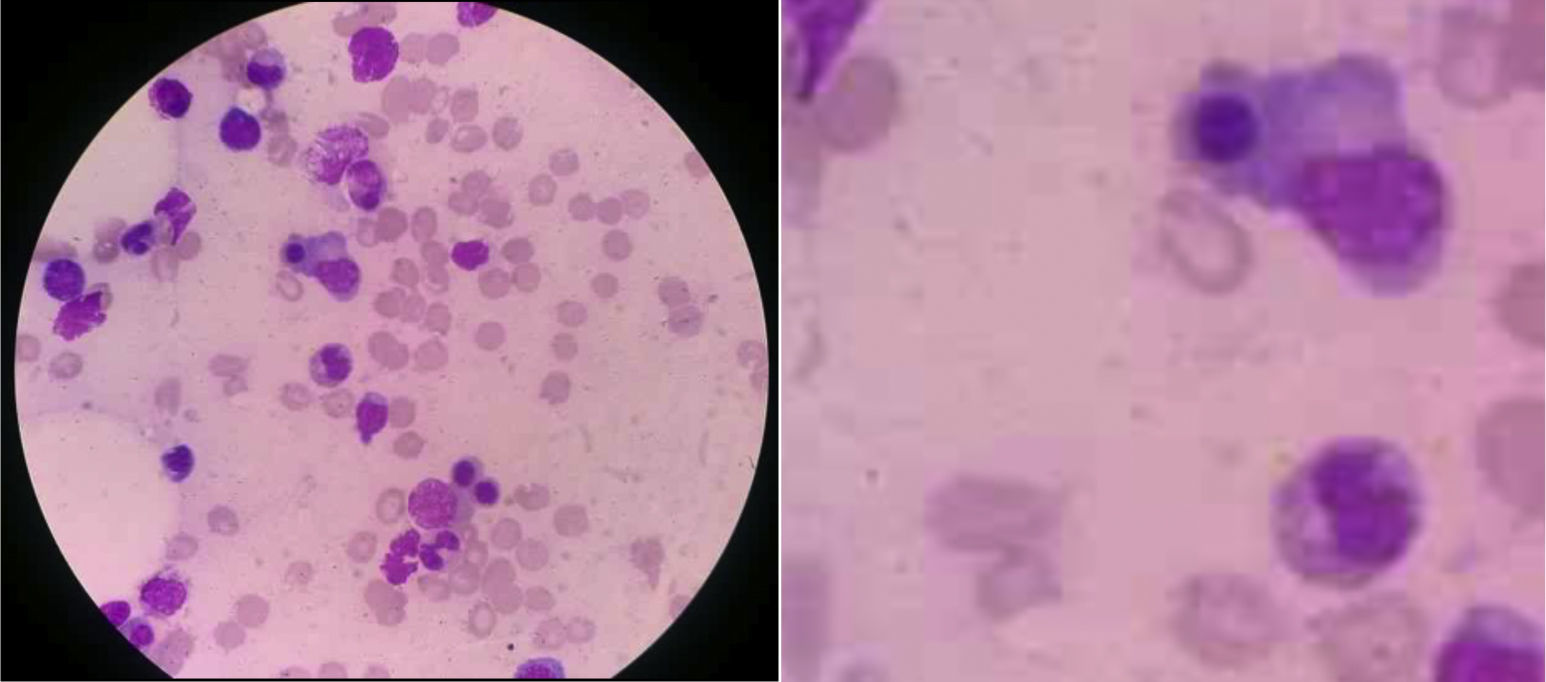
Treatment was begun with methylprednisolone and paracetamol, but the fever persisted. The suspicion of MAS led to tests that revealed hypertriglyceridemia (291mg/dL) and elevated ferritin (9579mg/mL). Myelogram disclosed bone marrow with spicules, activated macrophages with cytophagocytosis of cell debris and erythroblast nuclei (Fig. 1). Bacterial cultures of urine, blood and bone marrow were negative. It was decided to begin treatment with methylprednisolone pulses (1000mg/day for 3 days) plus cyclosporine A (2mg/kg body weight [bw]/day). The general status of the patient improved and the fever disappeared; however, the biochemical profile showed no changes. Thus, we started to administer intravenous etoposide (150mg/m2/day), the dose of cyclosporine A was increased (4mg/kg bw/day) and prednisone therapy was maintained (1mg/kg bw/day). The patient had a favorable clinical and biochemical course. Twelve days after receiving etoposide, she was asymptomatic and was discharged. Thereafter, we continued monitoring the patient for clinical and biochemical follow-up, which corroborated her improvement through the laboratory results (acute-phase reactants at normal levels).
Macrophage activation syndrome is associated with a high mortality rate (22%).2,3 For this reason, early diagnosis and proper treatment are essential to ensure the best possible prognosis. Etoposide, also referred to as VP-16, has an accelerated cellular mitosis and induces apoptosis. The cytotoxicity it generates causes alopecia, constipation, nausea, myelosuppression and secondary malignancy (leukemia); these effects are potentiated with the simultaneous use of cyclosporine A. For refractory HLH, it is recommended that there be an initial induction phase (2 weeks) with etoposide, cyclosporine A and dexamethasone,4,5 to be followed by 6 weeks of etoposide, if necessary.5 The utilization of this medication is still controversial, and there is a lack of consensus as to fully recommending its use because of paradoxical effects involving myelosuppression. This alternative is recommended only in refractory cases.5,6 Etoposide has been previously utilized in 8 patients with MAS and induced a favorable and rapid response in each case, with no adverse effects.7–9
In this instance, etoposide was added because the patient showed resistance to standard therapy. She received only 3 doses because of its high toxicity and the development of pancytopenia. The strategy we employed was successful; however, it proposes a strict control of the etoposide combined with the suggested treatment.
We presented MAS, a condition that is not very common, that was refractory to standard treatment. Thus, we opted for the use of etoposide. The patient we report progressed favorably. Nevertheless, as it is just a single case, we do not recommend generalizing this approach. The outcome can be taken into account in future studies that attempt to establish a complete scheme for the treatment of refractory reactive HLH.
Please cite this article as: Conde LF, Aedo KP, Miraval-Niño de Guzmán T. Síndrome de activación macrofágica: experiencia sobre el cuestionado papel del etopósido. Reumatol Clín. 2017;13:239–240.