An urgent search is currently underway for alternatives to antibiotics to prevent infections, due to the accelerated evolution and increase in antibiotic resistance. This problem is more serious for patients with recurrent infections, since they have to use many cycles of antibiotics per year, so the risk for antibiotic resistance is higher and can be life-threatening. In recent years, the use of prophylactic vaccines via the mucosal route for these patients with recurrent infections has been demonstrated as a potentially beneficial and safe alternative to prevent infections. The new knowledge about mucosal immunity and trained immunity, a form of innate immunity memory that can enhance the response to different infectious threads, has made it easier to extend its use. The application of the new concepts of trained immunity may explain the simultaneous pro-tolerogenic and boosting effect or effects of these drugs on diverse immune cells for different infections. In this review, we describe the immunomodulatory mechanisms of mucosal polybacterial vaccines and their connection with trained immunity and its utility in the prevention of recurrent infections in immunosuppressed patients.
Existe una búsqueda urgente de alternativas a los antibióticos para prevenir infecciones, debido al aumento acelerado de la resistencia a los antibióticos. Esto es más grave para los pacientes con infecciones recurrentes que tienen que ser tratados con varios ciclos de antibióticos al año, lo que incrementa el riesgo de resistencia a los antibióticos, que puede ser potencialmente mortal. En los últimos años se ha demostrado que el uso de vacunas profilácticas por vía mucosa para estos pacientes es una alternativa potencialmente beneficiosa y segura para prevenir infecciones. El nuevo conocimiento sobre la inmunidad de las mucosas y la inmunidad entrenada, una forma de memoria de la inmunidad innata que puede mejorar la respuesta a diferentes amenazas por infecciones, ha hecho más fácil expandir su utilización. La aplicación clínica de la inmunidad entrenada de estos fármacos podría explicar sus efectos simultáneos pro-tolerogénicos y potenciadores en diversas células inmunitarias para diferentes infecciones. En esta revisión describimos los mecanismos inmunomoduladores de las vacunas polibacterianas de la mucosa, su conexión con la inmunidad entrenada y su utilidad en la prevención de infecciones recurrentes en pacientes inmunocomprometidos.
According to the World Health Organisation, resistance to antibiotics is the main focus of attention in public health and the search for alternatives in prophylaxis to recurrent infections is urgent.1–3 Antibiotics are the keystone therapy for infectious diseases, they are effective against most bacterial, fungal and parasitic pathogens, but there is a growing problem of multi-resistance, and only a small number of drugs are effective against virus. One example is that despite the major reduction in respiratory and invasive diseases from Streptococcus pneumoniae (pneumococcal disease), due to the extended use of antibiotics and the introduction of new conjugated parenteral vaccines it is still a significant cause of morbimortality in humans.4,5 In this context of what has been called “the post-antibiotic era” there is a clear need for new alternative treatments for infectious diseases. Antibiotics will remain the main means of combating the pathogenic microorganisms, but alterative drugs to replace them or be used as adjuvant therapy to antibiotics may be efficacious in preventing these infections.
Under natural conditions, most bacteria do not live as individual cells, but as pseudomulticellular organisms which carry out commonly coordinated behaviour patterns through intercellular molecular signals in a process known as “Quorum sensing” (QS). This increases the capacity of the bacteria to adapt through absorption and incorporation, using the recombination of new genetic material of the bacterial community surrounding it.6 This multicellular coordination of bacterial attacks maximises the opportunities for establishing infection and allowing the bacteria to spread.7 Approximately 65% of the agents which cause human infections form biofilms which support chronic infections.7 The main benefit for the bacteria in forming these biofilms is that they are physically protected and possess between 10 and 1000 times more resistance than a single bacteria.7 Today biofilms present a major challenge to antibiotic efficacy.
One particular case is patients with recurrent bacterial infections who require almost continuous treatment with antibiotics, leading to an increased risk of antibiotic resistance. Furthermore, these patients frequently present with secondary dysbiosis due to repeated doses of antibiotics,8 which in turn may lead to a fungal superinfection. It is particularly in the context of recurrent infections that mucosal polybacterial vaccines could be a good alternative to wide-ranging antibiotics used for preventative ends. These vaccines are used as immunomodulators to prevent infections in immunocompetent patients, but their use in immunocompromised patients is increasingly greater.
Infection is one of the main causes of mobimortality in patients with systemic autoimmune diseases (SAD). Predisposition to infection in patients with SAD is associated with an anomalous response of the innate and underlying adaptive immune system to the actual disease and secondary to immunomodulators and immunosuppressants. Glucocorticoids may reduce the response of innate and adaptive immunity, inducing susceptibility to bacterial infections by streptococci, Staphylococcus aureus, gram negative bacilli, and diseases caused by fungi such as Candida and Aspergillus spp. Other immunosuppressant treatments may damage adaptive immunity inducing infections caused by virus (especially the herpes virus, such as herpes zoster, cytomegalovirus or Ebstein-Baar vurs), bacteria (Legionella spp., Listeria monocytogenes and Salmonella tiphy), Mycobacterium tuberculosis and fungi (Candida, Aspergillus, Histoplasma capsulatum and Cryptococcus) and Pneumocystis jirovecii. Moreover, B cell compromise with reduction in the production of immunoglobulins is associated with recurrent infections by encapsulated germs (Streptococcus pneumoniae, Haemophilus influenzae, meningococcus).
In the Spanish registry of adverse events of biological therapies (BIOBADASER) a higher rate of infections has been found in patients with rheumatoid arthritis (RA) who receive anti-TNF9; similar data are continuing to be confirmed in different reports, where the frequency of the infections and infestations amount to 23.9% of all adverse events recorded in patients with biologic therapy.
There are several possible explanations for a higher risk of infection in patients with RA. Recent evidence suggest that patients with RA have immunological changes which involve the majority of T cells circulating from an early stage during the course of the disease.10 As a result, the capacity of the immune system to respond to new antigenic stimuli may be compromised. Alternatively, therapy with glucocorticoids and other immunosuppressants may also predispose patients with RA to the development of sepsis.11,12 Other factors which may play a part in the risk of infection in patients with RA are factors relating to the disease (immobility, joint surgery), extra-articular RA manifestations (Felty syndrome, rheumatoid pulmonary disease) and comorbidities (diabetes mellitus).
The infections are usually located in the upper and lower respiratory tract, the skin and the genital and urinary tract. They are normally due to the Staphylococcus aureus and to gram negative bacteria. Equally, a higher frequency of herpes zoster has been reported and cases of opportunistic infections as well, such as listeriosis, widespread aspergilosis and other infrequent infections found in Spain, such as histoplasmosis and coccidioidomycosis, although rates were low. A higher rate of infections was also described with the other biologic agents.
As a result of the above, adjuvant or alternative therapeutic strategies to antibiotics are increasingly urgent, such as the immunisation of these patients with vaccines to prevent primary and recurrent infections, to reduce the repeated use of antibiotics, along with the appearance of resistance and of severe infectious diseases. In doing so, hospitalisations from these events will consequently drop.
This review covers the most up-to-date information on the mechanisms of action of mucosal polybacterial immunomodulators, also known as mucosal anti-infective vaccines.
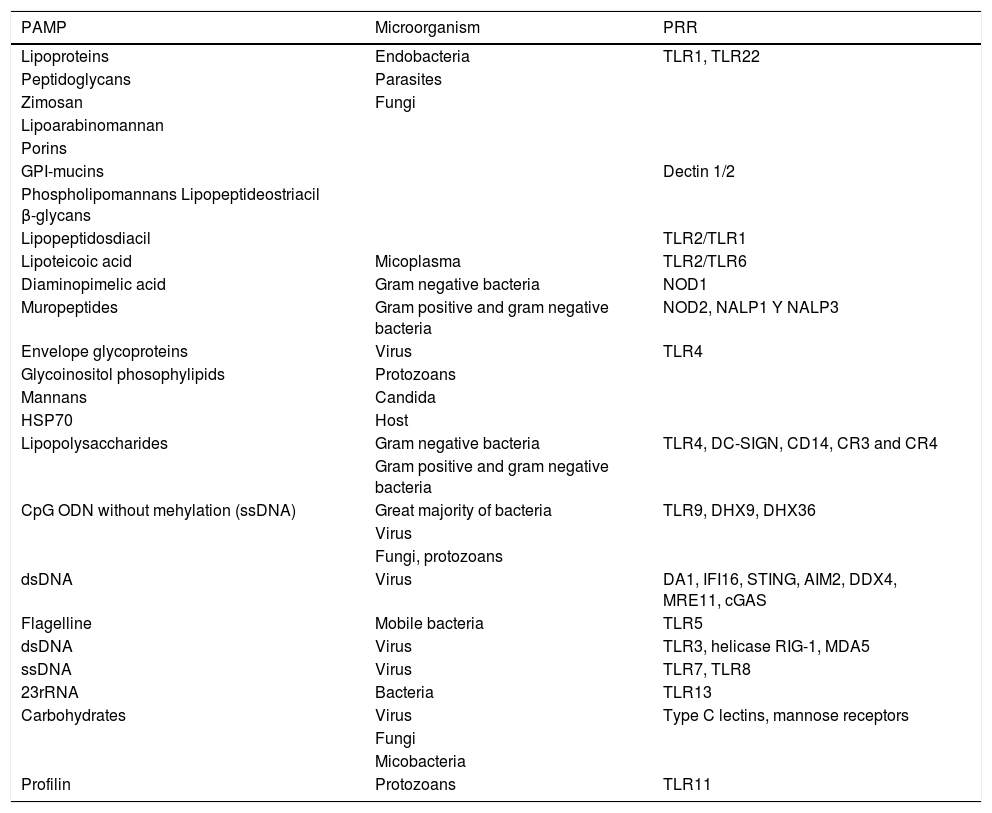
At the threshold: mucosal immunity, a new paradigm of trained immunityMucus membranes make up a very large surface area of our body, approximately 200 times greater than the area covered by the skin.13 The mucous membranes are the main entry route for infectious and environmental agents. As a result mucosal infections are one of the main causes of morbimortality in the world, with infants and the elderly being the most susceptible to them and no effective vaccines available in most cases. The mucosa-associated lymphoid tissue (MALT) encompasses a complex network of highly specialised and compartimentalised components of innate and adaptive immunity.13,14 It includes a nasopharangeal-associated lymphoid tissue (NALT), bronchial-associated lymphoid tissue (BALT), mucosa gastrointestinal-associated lymphoid tissue (GALT), urinary genital tract and a exocrine gland tissues.15,16 The dendritic cells (DC) are activated on recognizing common structures in the pathogenic microorganisms highly preserved throughout the evolution by pattern recognition receptors (PRR) (Table 1). DC antigen uptake may be carried out directly and indirectly.17 Indirect route is, for example, through uptake by M cells Microfold), by calciform cells, by neonatal Fc receptors (FcRn) and through apoptosis.17 M cells are specialised cells which capture antigens and enteric bacteria through the epithelial surface for uptake and processing by the DD and macrophages, which will initiate the effector immune response.18 The DC may also directly capture antigens from the lumen by extending their dendrites.18
Innate repertoire of pattern recognition receptors (PRR) which combine with pathogen associated molecular patterns (PAMP) and damage associated molecular patterns (DAMP).
| PAMP | Microorganism | PRR |
|---|---|---|
| Lipoproteins | Endobacteria | TLR1, TLR22 |
| Peptidoglycans | Parasites | |
| Zimosan | Fungi | |
| Lipoarabinomannan | ||
| Porins | ||
| GPI-mucins | Dectin 1/2 | |
| Phospholipomannans Lipopeptideostriacil β-glycans | ||
| Lipopeptidosdiacil | TLR2/TLR1 | |
| Lipoteicoic acid | Micoplasma | TLR2/TLR6 |
| Diaminopimelic acid | Gram negative bacteria | NOD1 |
| Muropeptides | Gram positive and gram negative bacteria | NOD2, NALP1 Y NALP3 |
| Envelope glycoproteins | Virus | TLR4 |
| Glycoinositol phosophylipids | Protozoans | |
| Mannans | Candida | |
| HSP70 | Host | |
| Lipopolysaccharides | Gram negative bacteria | TLR4, DC-SIGN, CD14, CR3 and CR4 |
| Gram positive and gram negative bacteria | ||
| CpG ODN without mehylation (ssDNA) | Great majority of bacteria | TLR9, DHX9, DHX36 |
| Virus | ||
| Fungi, protozoans | ||
| dsDNA | Virus | DA1, IFI16, STING, AIM2, DDX4, MRE11, cGAS |
| Flagelline | Mobile bacteria | TLR5 |
| dsDNA | Virus | TLR3, helicase RIG-1, MDA5 |
| ssDNA | Virus | TLR7, TLR8 |
| 23rRNA | Bacteria | TLR13 |
| Carbohydrates | Virus | Type C lectins, mannose receptors |
| Fungi | ||
| Micobacteria | ||
| Profilin | Protozoans | TLR11 |
Clinical and epidemiological evidence extracted from studies with standard vaccines highlighted a reduction of infant mortality in individuals vaccinated against measles and tuberculosis during their first year of life due to infection, compared with those who were not vaccinated.19 These results cannot be explained by a specific protection to these diseases, but due to a wide spectrum of protection, more in keeping with innate immunity, in what is called trained immunity.20,21 Trained immunity refers to the fact there are cells—for example monocyte/macrophage or natural killer (NK) cells, among others—capable of generating an intensified response to a second infection by the same micro organism and by a different (crossover protection) separate from the adaptive response (lymphocyte T and B), although it also increases the adaptive response.22–26 Studies from 50 years ago had already reported that infections confer antibacterial properties acquired through specific pathogenic bacteria and also others which are non-related.22 The mechanisms which measure this trained immunity include epigenetic and transcriptional reprogramming of cells of the innate immunity, including modifications in the histones and the DNA which may support the transcription of proinflammatory cytokines,23 a contribution of microRNAs, together with metabolic changes (Fig. 1).27
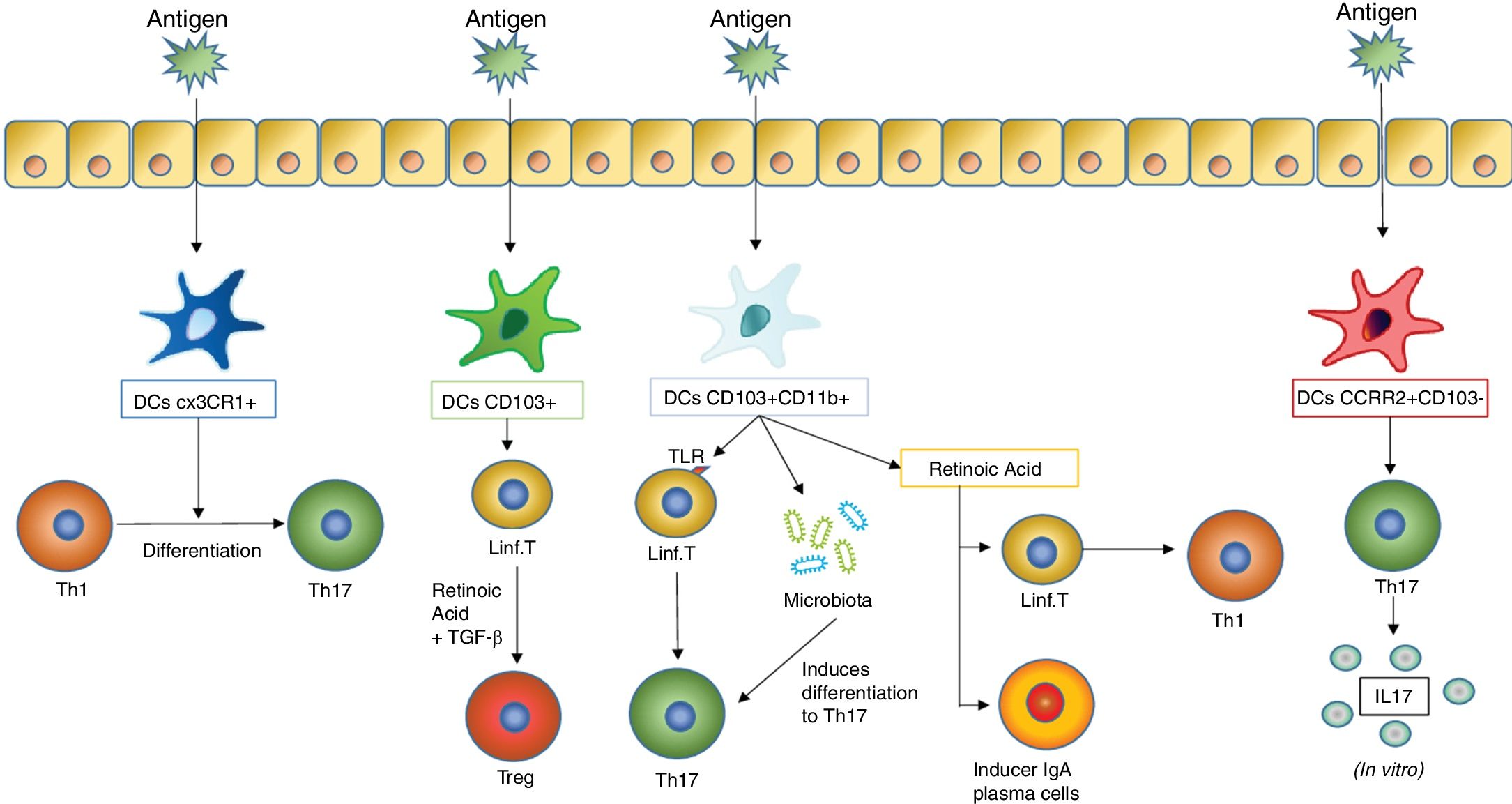
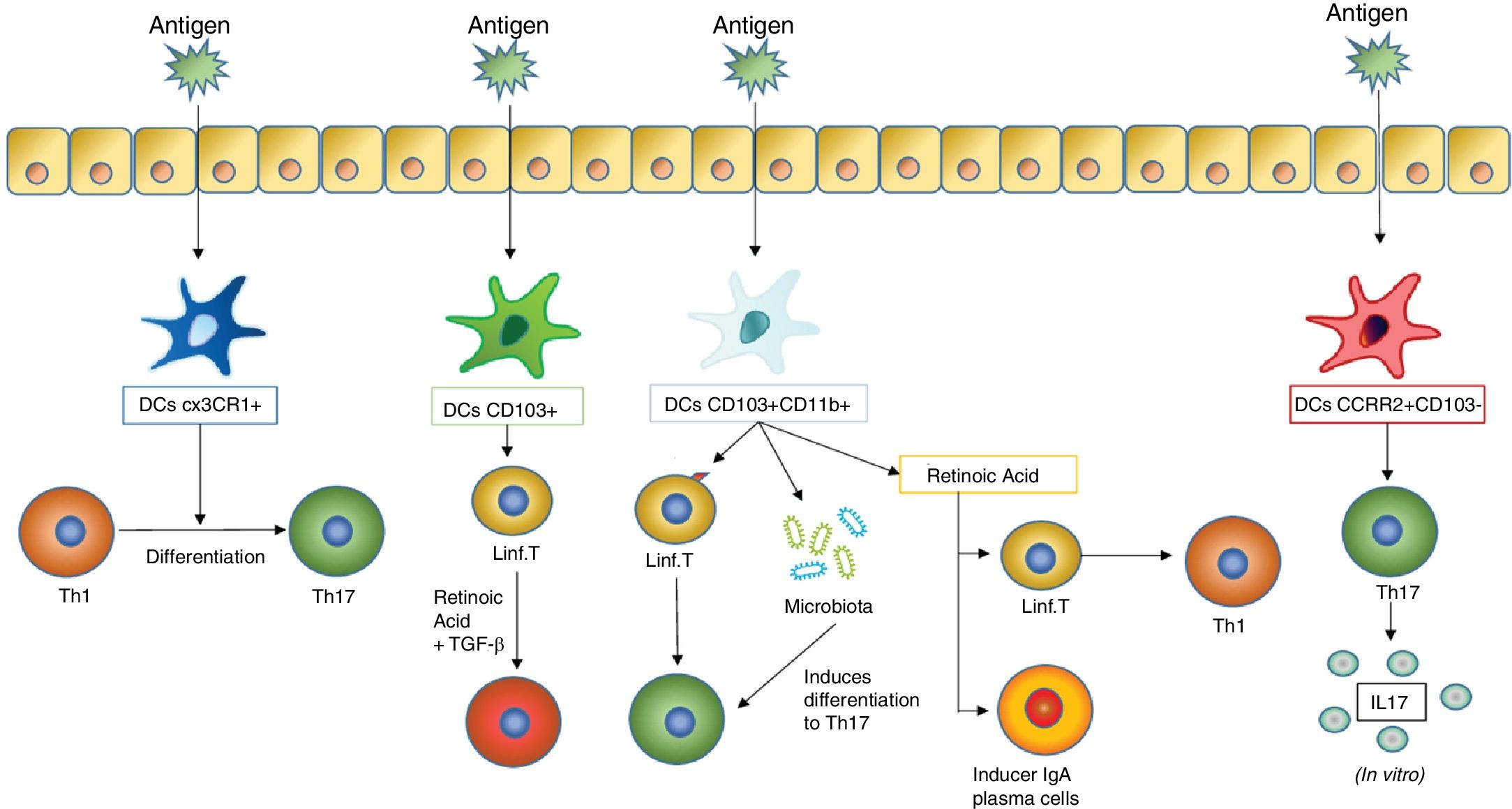
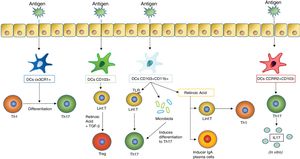
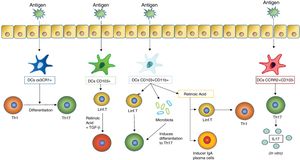
As they are stimulated with an antigen, the dentritic cells induce different responses in the mucosal tissues. One of the most important responses is the differentiation of T lymphocytes into different subtypes, which in turn depends on what type of dentritic cells stimulates this differentiation. The figure includes the previously mentioned DC subtypes which induce differentiation of T lymphocytes in the mucous membranes (such as DC CX3CR1+, CD103+ and CD103+ CD11b+) and those which induce a stimulation of T lymphocytes so that they produce cytokines (an action carried out by the DC CCR2+ CD103− which leads to the production of IL17 by T lymphocytes).
Mucosal anti-infective vaccines are complete inactive combinations of bacteria or fungi or bacterial lysates which stimulate the immune system through PRRs common to several germs, which according to many studies reduce the number of re-infections by the same pathogens and by other pathogens than the contents in the vaccines (Fig. 2). The underlying mechanisms to this collateral benefit may mostly be due to the “trained immunity” rather than any specific one, which it has also been seen contributes to the protector effect.
Trained immunity. Epigenetic reprogramming in innate immunity cells on stimulating the trained immunity. Once pathogen 1 had been recognised by a receptor, the monocytes experienced an epigenetic reprogramming (mainly through mutilations in the DNA) and metabolic changes, so that they were prepared to respond more robustly to a secondary non specific stimulation (pathogens 1, 2 and 3).
Unlike conventional vaccines administered parenterally, mucosal vaccines act directly on specific tissues where the pathogens start or spread infections. An immunological equilibrium exists on mucosal surfaces maintained by comensal flora. The induction of innate responses could lead to CD activation, macrophages, epithelial cells and NK cells, whilst stimulation from adaptive immunity would lead to the production of specific antibodies and B and T memory cells. Appropriately formulated mucosal vaccines stimulate the two components of the immune system, making them promising tools for therapeutic prevention and prophylaxis of infections.28–30 Anti-body responses in mucous membranes, principally secretory IgA, stimulated by these vaccines, inhibit a critical step in the microbial infectious pathogenesis, the adhesion of the micro organism to the epithelial cells of the mucous membrane, conferring better protection against colonisation and invasion than parenteral vaccines.31 The mucous membrane IgG mark the opsonization and internalisation of pathogens which have crossed over the epithelial barrier through phagocytosis. This leads to activation of the phagocytes and elimination of the pathogens or the presentation of antigens derived from them, encouraging the differentiation of multiple subtypes of T effector lymphocytes, such as lymphocytes Th17, lymphocytes Tγδ and lymphocytes T CD8+cytotoxics (CTL), for the destruction of the pathogen.32 The tissue-resident memory T cells (TRM) may respond quickly to a pathogen-local re-infection, regardless of T lymphocyte recruitment from the blood. When the TRM CD8+ are activated they release IFN-γ, which amplifies and complements the innate immune response activation creating an antiviral microenvironment in the mucous membrane. The induction of TRM lymphocytes is currently a challenge for vaccination strategies, and mucosal vaccines may be an effective route for persistently generating TRM lymphocytes.33
Administration through mucous membranes induces tolerance, which is an added benefit in SAD treatment. Sublingual vaccines for allergenic immunotherapy have been used for one hundred years and recent studies suggest that sublingual immunotherapy may improve induction of systemic tolerance. In accordance with the current model of underlying immune mechanisms, the allergen is captured by the tolerogenic plasmacytoid CD of the tonsils which later emigrate to the cervical lymphatic nodules and induce a response of the Treg lymphocytes.34 Biopsies of oral epithelium have shown that there is a larger amount of Treg cells in patients treated with sublingual immunotherapy than in patients treated with placebo.35 This model in allergy would be transferable to that which occurs in patients with SAD, although specific studies are required to prove this.
Anti-infective vaccines in autoimmune diseasesAutoimmune diseases are a key objective for the development of these drugs.36 The induction of secretion of specific sIgA has many regulatory mechanisms which may also promote a low T lymphocyte mucosal and systemic response capacity, through a modulating effect of the differentiation of Treg lymphocytes.37
Experimental modelsIntranasal and sublingual mucosal administration of antigens may induce deep suppression of cellular immunity, as has been shown by the reduction of delayed hypersensitivity reactions, low levels of T cell proliferation and high levels of immunosuppressant cytokine secretion.34,38,39 A recent study demonstrated that the oral vaccine with an attenuated vaccine of Salmonella would induce specific Treg cells to the antigen I of the colonisation factor (CFA/I) of the fimbrias, whilst simultaneously protecting against experimental autoimmune encephalomyelitis (EAE), a murine model of multiple sclerosis.40 In this study, oral vaccination compared with an irrelevant antigen would induce Treg cells which could control autoimmunity and a concomitant anti-infectious effect. Another study in EAE has proven this dual effect against a respiratory tract bacterial infection through the production of the anti-inflammatory cytokine IL-10 which would eliminate the migration of auto-reactive T cells from the central nervous system.41 Similar studies in the experimental model of arthritis induced by collagen in DBA/1 mice suggest that the induction of a bystander immunity against the bacteria could potentially control autoimmune or inflammatory processes.36,38 This immunomodulating effect of some bacteria could be exploited to improve a new concept of bacterial mucosal vaccines as a therapeutic innovation to boost immunological tolerance (cross-over tolerance) and homeostasis within the context of autoimmune diseases.
Clinical trialsOne prospective observational clinical trial evaluated the clinical and immunological effects of treatment with sublingual vaccines of bacterial combinations in antigen specific responses to bacteria which were responsible for respiratory tract infections.42 Daily immunisation with a polyvalent bacterial sublingual preparation for a period of 6 months was studied in a cohort of 17 patients with recurrent respiratory infections (RRI). The number of respiratory infections in immunised patients significantly dropped compared with the previous year. There was also a clear reduction in the severity and duration of the infectious respiratory events. The immunological results demonstrated a considerable increase in the prolipherative frequency and capacity of specific T CD4+ lymphocytes in blood compared with bacterial antigen contents in the vaccine, after 6 months of treatment. Furthermore, an increase in the prolipherative capacity of the specific T CD4+ and CD8+ lymphoctyes of influenza virus antigens after 6 months of treatment was observed (not contained in the vaccine), which may suggest that the bacterial preparation had stimulated the T lymphocytes non specifically in vivo to the influenza virus, which led to a more powerful response. The case of one of the patients described in this study is interesting. The patient had suffered 12 episodes of oral herpes the previous year and only reported 3 episodes after immunisation with the bacterial preparation, suggesting a non specific in vivo effect which enhanced the antiviral response (trained immunity). In another prospective observational study with 669 women with recurrent urinary tract infections (RUTI) efficacy was compared between a prophylactic treatment with antibiotics (Septrin) and the bacterial sublingual preparation, where 100% of patients experienced a new infection the following year versus less than 10% in the group treated with the bacterial preparation. An interesting finding in this study was that the patients who had previously had infections with bacteria which were not included in the vaccine did not develop reinfections, suggesting that a horizontal or cross protection could be attributed to a strengthening of the innate trained immunity.43–45
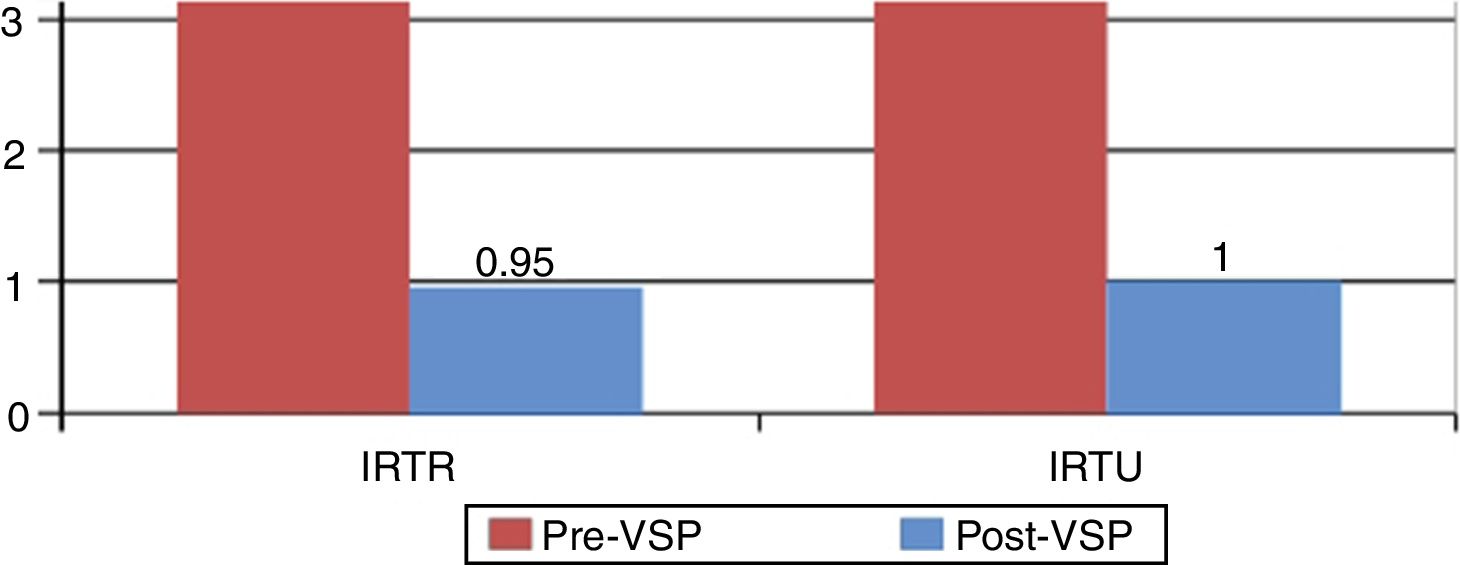
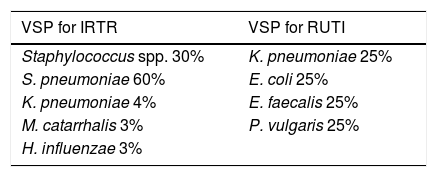
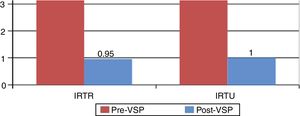
In one prospective observational study performed by our group 50 patients with inflammatory diseases (RA [43.18%], systemic erythematous lupus [22.72%] and mixed connective tissue disease [6.81%]) under treatment with DMARD biologics and/or non biologics and glucocorticoids mostly at low doses, who presented with recurrent urinary and/or respiratory infections, were treated with two different polybacterial sublingual preparations depending on the type of infection (Table 2). The vaccines were administered in cycles of 3 months per year and the clinical response was recorded after 6 months and after 1 year. Paired comparison of the number of infectious events in both groups demonstrated a considerable reduction in the rate of repetitive urinary tract infections (RUTI) of 5.57±8.41 compared with 1.0±1.28 (P<.05) and in the rate of repeated respiratory tract infections (RRTI) of 4.1±1.98 compared with .95±1.52 (P<.05) in the previous year to the year after the vaccine, as well as a considerable reduction in the use of antibiotics 6 months after the vaccine (Fig. 3). Six of the 38 patients treated with the vaccine for RUTI who presented simultaneously with respiratory infections improved significantly not just from their urinary infection but also from the respiratory infection, suggesting an innate cross-over protection.46,47
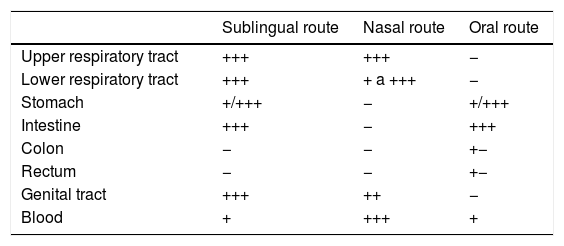
Therapeutic interventions with mucosal anti-infective vaccines have different advantages to standard parenteral vaccines: (a) their effects are directly felt in the infection site of the mucous membrane and they may prevent infection and colonisation by pathogens; (b) immunisation in mucous membranes may lead to the secretion of antibodies in other more distant mucous membranes and also systemically10; (c) administration does not require medical professionals and they may be used in mass vaccines and disease prevention campaigns, (d) they are painless and simple to administer. Also, bearing in mind that gram positive bacteria stimulate the production of IL-12 and that gram negative bacteria stimulate the production of IL-10 by monocytes, the combination of gram positive and gram negative bacteria in anti-infective vaccines of mucous membranes appears to carry a synergic immunological response which could be greater depending on the pathology involved.48 Differences in the induction of immunity response were observed depending on the administration route, with sublingual being the most advantageous (Table 3). Lastly, there was a huge potential for development taking advantage of these trained immunity characteristics, which would be exploitable and improbable through tools such as reverse vaccinology for the development of adjuvants to standard vaccines. Notwithstanding, -unlike parenteral vaccines, these vaccines require repetitive stimulus and the duration of active treatment may be long and requires patient adherence to it.
Comparison of anatomical dissemination of sIgA depending on the immunisation routes.
| Sublingual route | Nasal route | Oral route | |
|---|---|---|---|
| Upper respiratory tract | +++ | +++ | − |
| Lower respiratory tract | +++ | + a +++ | − |
| Stomach | +/+++ | − | +/+++ |
| Intestine | +++ | − | +++ |
| Colon | − | − | +− |
| Rectum | − | − | +− |
| Genital tract | +++ | ++ | − |
| Blood | + | +++ | + |
To sum up, therapeutic intervention with polybacterial preparations of mucosal administration with inactivated complete bacteria or with bacterial lysates stimulate the innate and adaptive immunity which is present in the mucous membranes49 and offers greater clinical benefits, with its effect being attributed to the following mechanisms: (a) complete bacteria are presented to the immune system in the most natural way, maximising their entire potential as immunogens,45,50,51 and (b) the complete bacteria stimulate different immune mechanisms, which have been confirmed as highly important for a complete cellular activation, such as phagocytosis.52 A change of design in the outcome of these vaccines is required, based on a clinical benefit effect rather than on the specific antigen itself.
Immunodeficiencies secondary to immunosuppressant therapy (organ transplant, rheumatological disorders, oncology, and organ specific autoimmune diseases), chronic metabolic diseases (diabetes) or chronic infections (HIV) are situations which also lead to recurrent infections. The use of polybacterial vaccines in actively immunosuppressed patients for preserving a transplanted organ, or for keeping an autoimmune disease under control is a genuine clinical challenge due to the high risk of infections and the subsequent impact on morbimortality.
ConclusionsTreatment with polybacterial drugs or mucosal anti-infective vaccines which enhance the innate trained immunity as the main objective in the prevention of recurrent infections is an innovative therapeutic strategy with promising results. New prospective studies and randomised clinical trials are required to assess their efficacy and optimise current preparations. Also, since the purpose of these drugs is a broad spectrum protection, the primary objectives of efficacy must be designed in keeping with clinical response.
FinancingThis research study did not receive any specific finance from public sector agencies, the trade sector or any non profit-making entities.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Sánchez Ramón S, Manzanares M, Candelas G. Vacunas antiinfecciosas de mucosas en la profilaxis de infecciones recurrentes: más allá de las vacunas convencionales. Reumatol Clin. 2020;16:49–55.