Primary Sjögren's syndrome is a chronic systemic autoimmune disease that causes destruction of lacrimal and salivary glands. The most common and earliest symptoms are oral and ocular dryness. Dry mouth makes talking difficult, tasting and chewing properly, impairing quality of life of these patients. The most common oral signs and symptoms are hyposialia with or without xerostomia, tooth decay, fungal infections, traumatic oral lesions, dysphagia, dysgeusia, and inflammation of salivary glands. There are different therapeutic strategies, depending on the severity of each case, and the increase in the amount of saliva, to reduce the number of cavities and oral infections. It is particularly important to establish a close relationship between the dentist and the rheumatologist in order to make an early and correct diagnosis, promoting appropriate dietary and hygiene measures, as well as to treat and prevent potential oral complications.
El síndrome de Sjögren primario (SSp) es una enfermedad autoinmune sistémica crónica, que cursa con destrucción del tejido glandular lagrimal y salival. Sus síntomas más frecuentes y tempranos son la sequedad oral y ocular. La sequedad oral dificulta que el paciente hable, deguste y mastique correctamente, lo que disminuye la calidad de vida del enfermo. Los signos y síntomas orales más frecuentes son la hiposialia con o sin xerostomía, la caries dental, las infecciones fúngicas, las lesiones orales traumáticas, la disfagia, la disgeusia y la inflamación de las glándulas salivales. Existen distintas estrategias terapéuticas en función de la gravedad de cada caso que aumentan la cantidad de saliva y disminuyen el número de caries e infecciones orales. Por ello, es de especial importancia establecer una relación cercana entre el dentista y el reumatólogo que permita hacer un diagnóstico temprano y correcto, fomentar las medidas dietéticas e higiénicas adecuadas, tratar y prevenir las posibles complicaciones orales.
Primary Sjögren's syndrome (pSS) is a chronic, systemic, autoimmune, rheumatologic disease characterized by the presence of a lymphocytic inflammatory infiltrate in the salivary and lacrimal glands, which results in the destruction of the gland tissue. The most common and earliest symptoms of this disease are dry eyes and mouth, although extraglandular manifestations involving musculoskeletal, pulmonary, gastrointestinal, hematological, cutaneous, renal and neurological systems can also develop.1,2
Primary Sjögren's syndrome was described for the first time by the Swedish physician, Henrik Sjögren, who reported the cases of 19 women presenting with ocular dryness, the great majority of whom had rheumatoid arthritis.3 There are 2 types of Sjögren's syndrome, pSS, which occurs as an isolated disease, and secondary (sSS), which is associated with other autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus.2,4 The pathogenesis of pSS has been related to immunological, inflammatory, genetic, epigenetic, environmental, hormonal and infectious factors.5
The prevalence of pSS ranges from 0.5% to 4% of the general adult population. It is diagnosed more frequently in women than in men, in a proportion of 9:1, and it usually presents between the fourth and sixth decade of life, although it can appear at any age.2,4,6,7 It is widely reported throughout the world, although it is most prevalent among Caucasians. As there are few symptoms, especially in the early stages, the disease is underreported, and there can be a considerable delay in the diagnosis, with a mean interval between symptom onset and definitive diagnosis of 3.5 years.7
The classification criteria for pSS include clinical signs and objective tests to detect dry eye and mouth, characteristic serological abnormalities (presence of anti-Ro/SSA and/or anti-La/SSB), and compatible histopathological findings in minor salivary glands (focal lymphocytic sialadenitis, with a focus score ≥1; a focus is defined as an aggregate of at least 50 lymphocytes and the score, as the number of such foci in a surface area of 4mm2 of salivary gland tissue).8 At the present time, the classification criteria most widely employed are those proposed by the American-European Consensus Group in 2002 (Table 1).9 The American College of Rheumatology and the Sjögren's International Collaborative Clinical Alliance have recently drawn up new classification criteria (Table 2).1,10
Diagnostic Criteria for Primary Sjögren's Syndrome Proposed by the American-European Consensus Group.
| Ocular symptoms: a positive response to at least one of the following questions:Have you had daily, persistent, troublesome dry eyes for at least 3 months?Do you have a recurrent sensation of sand or gravel in the eyes?Do you use artificial tears more than 3 times a day?Oral symptoms: a positive response to at least one of the following questions:Have you had a daily feeling of dry mouth for at least 3 months?Do you had recurrent or persistent swelling of the salivary glands?Do you frequently drink liquids to aid in swallowing dry food?Ocular signs, that is, objective evidence of ocular involvement defined as a positive result for at least one of the following tests:Schirmer's I test, performed without anesthesia (<5mm in 5min)Rose bengal score or other ocular dye score (>4 according to van Bijsterveld's scoring system)Histopathology of minor salivary glands (obtained using apparently healthy mucosa): focal lymphocytic sialoadenitis, with a focus score≥1; a focus is defined as an aggregate of at least 50 lymphocytes and the score, as the number of such foci in a surface area of 4mm2 of minor salivary glandSalivary gland involvement, objective evidence of salivary gland involvement defined by a positive result for at least one of the following diagnostic tests:Unstimulated whole salivary flow (<1.5ml in 15min)Parotid sialography showing the presence of diffuse sialectasias (punctate, cavitary or destructive pattern), with no evidence of obstruction in the major ducts, according to the scoring system of Rubin and HoltSalivary scintigraphy showing a reduced concentration or delayed excretion of the tracer, according to the method proposed by Schall et al.Autoantibodies: presence in serum of the following autoantibodies:Antibodies to Ro(SSA) or La(SSB) antigens, or bothRevised guidelines for the classification of primary Sjögren's syndrome: in patients with no potentially associated disease, primary Sjögren's syndrome should be defined as follows:The presence of any 4 of the above 6 items indicating primary Sjögren's syndrome, as long as item 4 (histopathology) and 6 (serology) are positiveThe presence of any 3 of the 4 objective criteria (for example, items 3, 4, 5 and 6)The classification tree procedure represents a valid alternative method for classification, although it should be properly used in clinical-epidemiological studies |
Diagnostic Criteria for Primary Sjögren's Syndrome Proposed by the American College of Rheumatology and Sjögren's International Collaborative Clinical Alliance.
| According to this group, patients with Sjögren's syndrome should meet 2 of the following 3 conditions:Presence of anti-Ro/SSA and/or anti-La/SSB or presence of rheumatoid factor and ANA≥1:320Keratoconjunctivitis sicca with ocular staining score of 3 or higher (provided the individual is not currently using eye drops for glaucoma and has not had corneal surgery or cosmetic eyelid surgery during the preceding 5 years)Minor salivary gland biopsy revealing focal lymphocytic sialadenitis with a focus score greater than 1 per 4mm2 of gland tissue |
The majority of the patients with pSS first note dryness in the mouth and/or eyes. Mouth dryness makes it difficult to speak correctly and to taste and chew food properly. Dry mouth is frequently the first complaint of these patients, who constantly need to chew gum or suck on hard candy to stimulate the production of saliva, and who wake up several times a night to drink large amounts of water.2,11 Thus, dry mouth has an important impact on the quality of life of patients with pSS.12–14
Individuals with dry mouth usually consult first with their primary care physicians, who, in many cases, do not have the means to determine whether the dryness reported by the patient is real (hyposalivation) or subjective (xerostomia). It is essential to establish this difference because many individuals of advanced age, mostly women, have burning mouth syndrome (BMS), a complex condition that produces, among other symptoms, a subjective sensation of dry mouth that cannot be detected in supplemental tests. Burning mouth syndrome is characterized by the presence of a burning sensation in the oral mucosa, despite normal analytical findings and no decrease in salivary flow. It is encountered most often in women from the age of 40 years on. Its prevalence ranges between 0.7% and 7%, and is higher among postmenopausal women, in whom the prevalences is as high as 12%–18%. This syndrome frequently affects the tip and lateral edges of the tongue, lips, and hard and soft palate. Patients with BMS also experience pain, loss of taste sensitivity and xerostomia.15–17
It is also necessary to rule out other possible causes of dry mouth, such as alterations in afferent stimuli, central nervous system disorders, dysfunction of the afferent pathways of the autonomic nervous system, chronic salivary gland inflammation (of immunological and nonimmunological origin), use of drugs associated with xerostomy18 (Table 3), psychological disorders, use of tobacco and other drugs, systemic diseases, treatment with head and neck radiotherapy and dehydration.4 If the primary care physician findings no modifiable cause associated with xerostomia, the patient should be referred to a dental professional for a complete check-up; if there is any datum in the patient's history or finding in the physical examination that suggests Sjögren's syndrome, the patient should also be referred to a rheumatologist.
Drugs Associated With the Presence of Xerostomia.
| Anorexic agentsAnxiolyticsAnticonvulsantsTricyclic antidepressantsAntiemeticsAntihistaminicsAntiparkinsonian drugsAntipsychoticsBronchodilatorsDecongestantsDiureticsMuscle relaxantsNarcotic analgesicsSedativesAntihypertensive drugsAntiarthritic agents |
On other occasions, the first specialist consulted by a patient with dry mouth is his or her dentist. The dentist should determine whether the patient has hyposalivation by recording a thorough clinical history, which should include, among other tools, a questionnaire on dry mouth that reflects how xerostomia affects the quality of life of the patient12–14; this will document all the symptoms reported by the patient and the medications he or she takes. In addition, the salivary flow should be measured (sialometry)15 and the severity of the mouth dryness as perceived by the patient should be quantified using a visual analog scale.7
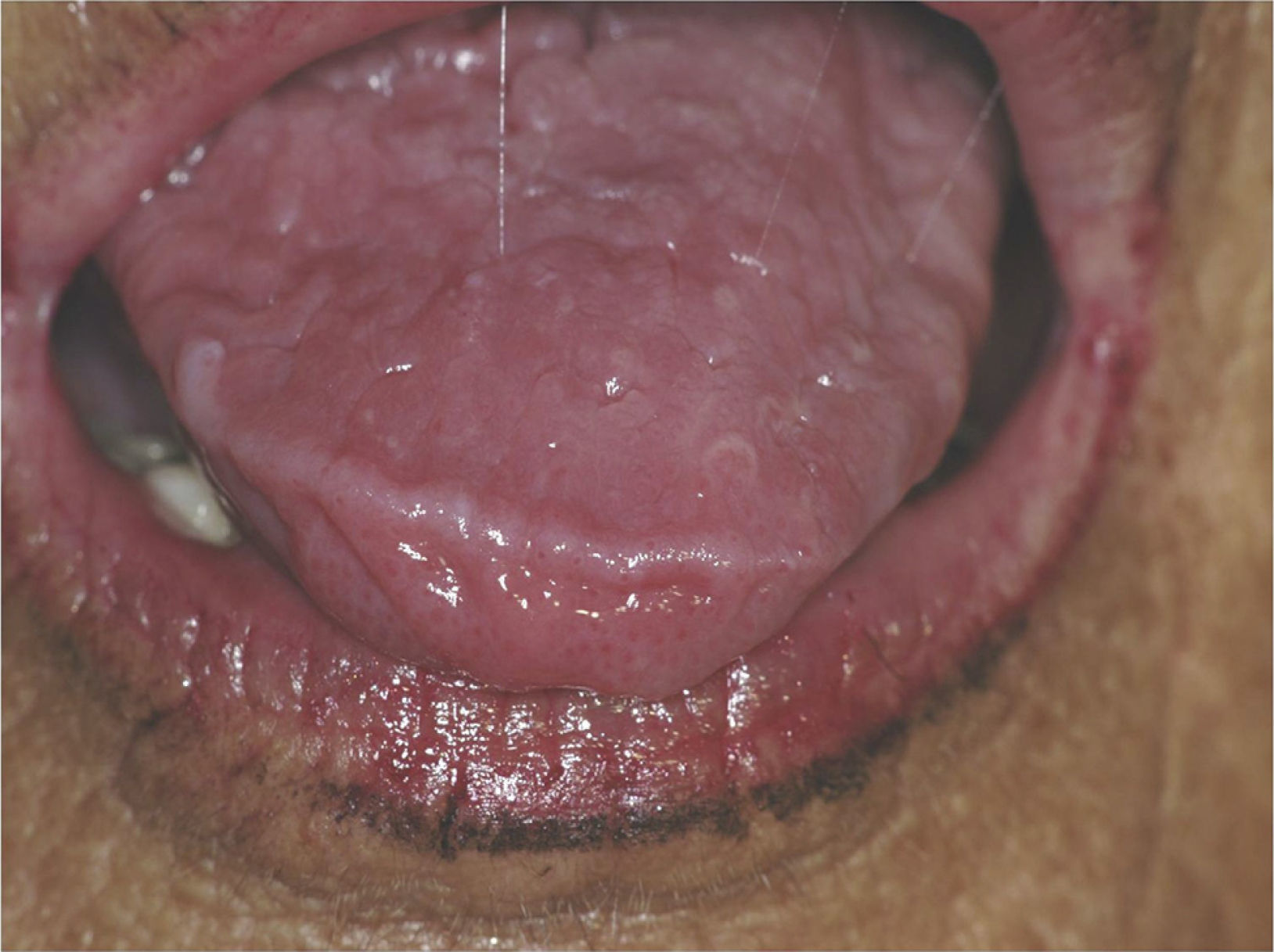
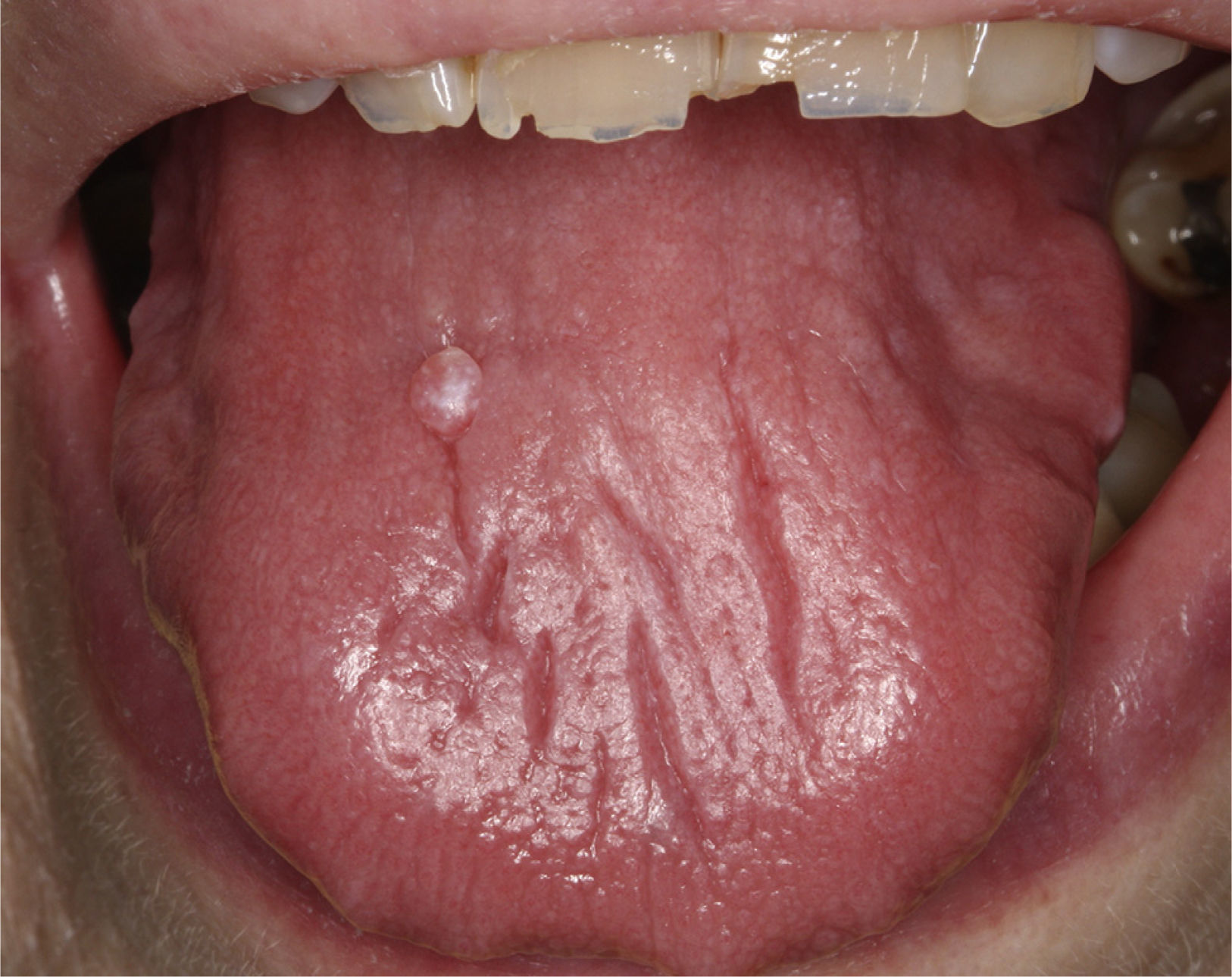
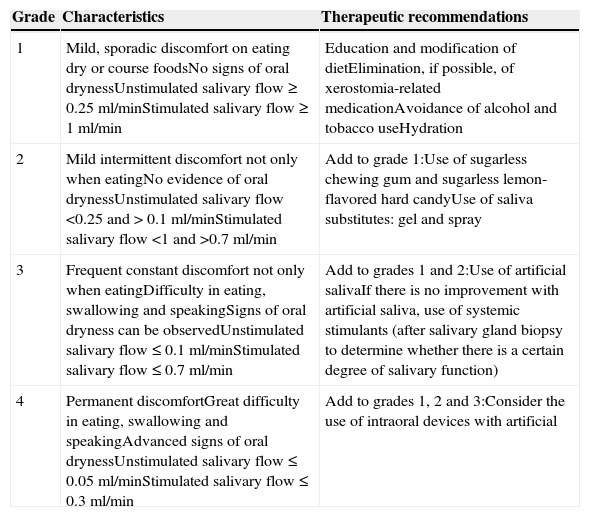
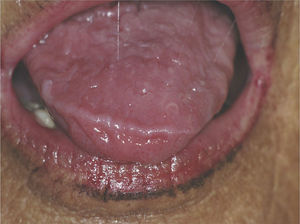
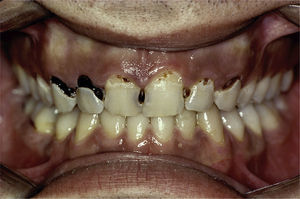
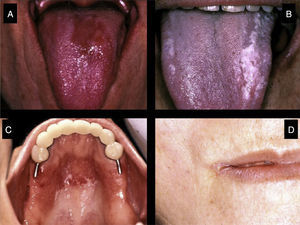
Certain data from the clinical interview point toward hyposalivation: difficulty in speaking, chewing and swallowing, altered taste and a constant need to drink water (even at night). Likewise, there is a series of clinical signs associated with hyposalivation: the presence of dry, fissured lips and a coated, erythematous and fissured tongue. It is also common to find stringy saliva (Fig. 1), angular cheilitis, rampant caries in atypical locations (Figs. 2 and 3), occlusal wear, gland inflammation, mucositis and oral ulcers.7 When the dentist detects the presence of hyposalivation and can find no local cause, in addition to attempting to alleviate the symptoms, he or she should refer the patient, together with a detailed report, to the primary care physician who, in turn, should refer the patient to the rheumatologist for the study of the systemic disease.
The majority of the oral manifestations encountered in patients with pSS are the consequence of the hypofunction of the salivary glands.16,17 In the following subsections, we describe, separately and in detail, each of the oral signs and symptoms that can be observed in patients with pSS (Table 4).
The 3 pairs of major salivary glands (parotid, submandibular and sublingual) account for 90% of the whole saliva output.16 Adults normally produce saliva at a rate of 0.4ml/min or 1–1.5l/day.4,17 Salivary output is a regulated process. An increased saliva production is achieved through the stimulation of chemical and/or mechanical processes, basically chewing and food intake; between meals, the production decreases, reaching nearly zero during sleep.4
Saliva is basically composed of water, but also contains electrolytes, proteins, glycoproteins, enzymes, like defensins, proteases, histatins and lysozymes, and other molecules with biological and biochemical properties that are essential for the maintenance of the physiology of the oral environment. The microbiological composition of saliva plays a fundamental role; in patients with decreased salivary flow, there is an alteration of the composition of the oral bacterial plaque. The total bacterial count in saliva in patients with pSS is similar to that of control patients, but a decrease in salivary flow is related to an increase in the concentration of certain microorganisms, such as Lactobacillus acidophilus, Streptococcus mutans and Candida albicans, which may be the origin of a higher incidence of caries and candidiasis in these patients. In contrast, the microorganisms related to periodontal disease are not more abundant in patients with pSS. Thus, there has been no evidence of an increase in periodontitis in these patients.4,17
Saliva facilitates correct speech, lubricates the mucosa, has a buffer or barrier effect, remineralizes the tooth enamel, helps to taste food and to form the food bolus, initiates the process of digestion and has defense mechanisms that protect against infections.17 Likewise, it forms a coating on the hard and soft tissues of the oral cavity that protects and maintains the moisture, not only of the mouth, but of the oropharynx and esophagus as well. Moreover, the components of saliva act as buffers against changes in pH in the oral cavity, and salivary proteins can be absorbed by the tooth surfaces, forming an organic film in which the demineralization and remineralization of dental tissues take place.4 Thus, the decrease in saliva production in patients with pSS results in the development of caries, infection (candidiasis), a burning sensation in the mouth, glossodynia (a burning sensation in the tongue), dysphagia, dysgeusia (an alteration in the sense of taste), difficulty in speaking correctly, oral lesions, salivary gland inflammation and gastroesophageal reflux.6,17
CariesCaries are due to the breakdown of the hard tissues of the teeth by the acids produced by dental plaque. There are many factors associated with a higher incidence of caries, such as diet (composition and eating frequency), hygiene, education and awareness of the problem, health expectations and the characteristics of the saliva (amount, composition, buffering capacity, capacity for oral sugar clearance, fluoride concentration, etc.).4,17 Patients with hyposalivation also have a decreased secretion of immunoglobulin A, an antibody responsible for the oral mucosal immunity that prevents dental caries.17
The force of the salivary flow and the action of the tongue, cheeks and lips on the teeth mobilize the bacteria in the cavity. In patients with pSS, food gets trapped on the vestibular surfaces of the teeth due to the poor lubrication and the lack of mechanical action of the saliva.19
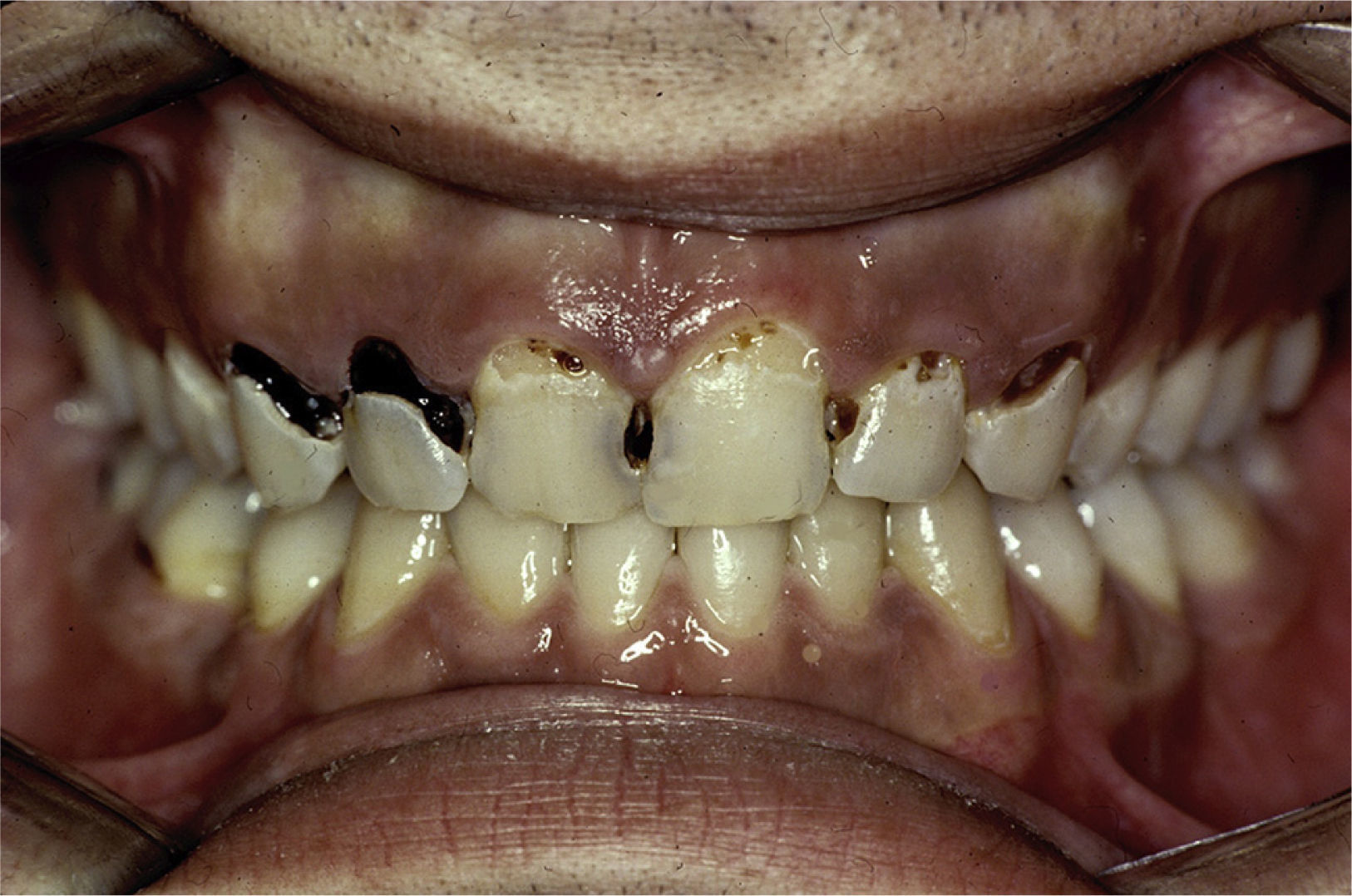
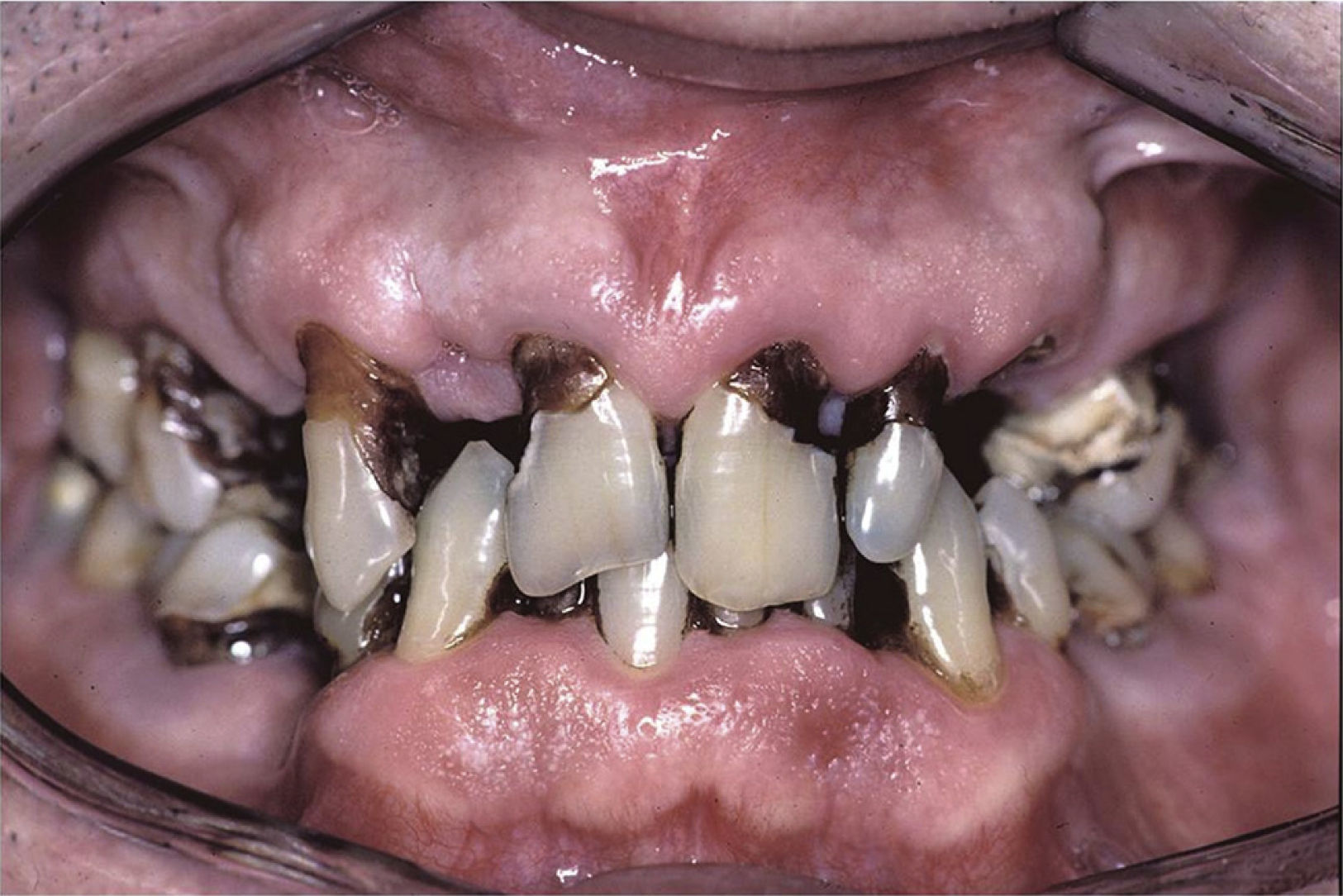
These patients have a higher incidence of caries and, moreover, the caries appear on the surface, near the root of the tooth and at other atypical sites like the lingual surface, incisal edge and cusp of the teeth (Figs. 2 and 3).4,6,17 In addition, the regulation of the salivary pH is poorer in these patients. For example, after the consumption of sweets, the pH is lowered; however, the saliva of individuals with pSS does not act as an effective buffer and, thus, their risk of caries increases.4 The greater number of caries means that these patients visit the dentist more often, lose more teeth and have more restored teeth in comparison with control subjects.20
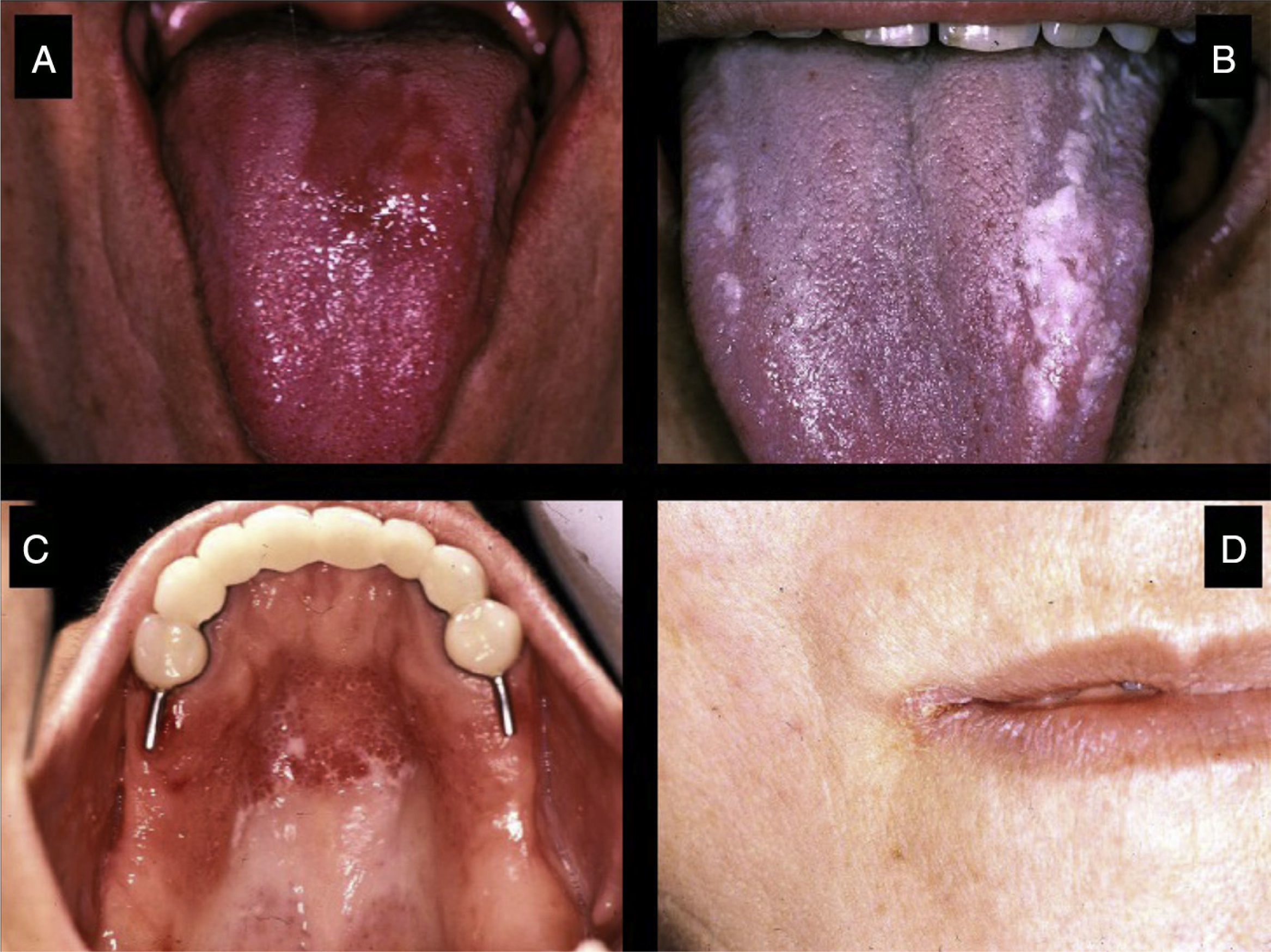
Fungal InfectionsPatients with pSS have a higher prevalence of oral fungal infections, mainly candidiasis.6,21 This infection occurs due to the reduction in the output of saliva and in its buffering capacity. The clinical presentation of oral candidiasis can take several forms: erythematous lesions, candidiasis affecting the mucosa covered by the prostheses of denture-wearers and pseudomembranous candidiasis (Fig. 4). At extraoral sites, it can lead to angular cheilitis, which is characterized by dry, fissured, erythematous lesions in the corners of the mouth.6,17 Chronic erythematous candidiasis, mainly involving the tongue, palate and corners of the mouth, can affect up to 70%–80% of the patients with pSS.7,17,22
Other Lesions of the Oral MucosaThe saliva plays a fundamental role in the lubrication of the oral mucosa, preventing damage due to trauma or friction. Patients with pSS can develop traumatic lesions after eating foods that are coarse or abrasive, like toast or potato chips, due to the dryness of the mucosa.6,17
Individuals with pSS can have dry, fissured lips and lingual depapillation. The tongues of patients with hyposalivation look like ground meat, and are dry, fissured and sticky to the touch.17,20,22 In addition, these patients usually tolerate poorly their partial or complete dentures, since saliva forms a film that aids in the retention of these prostheses and, when the output is reduced, the stability is weakened. In turn, dentures usually produce traumatic lesions.6,17,23
Orofacial PainThe symptom that normally accompanies xerostomia in pSS is the burning sensation in the mouth, or oral burning, also referred to as glossodynia. In these patients, this sensation is mainly due to hyposalivation, and can become worse if they develop candidiasis or take drugs that favor the reduction in salivary output. In these cases, it is important to perform a differential diagnosis involving other conditions that produce an oral burning sensation such as anemia, oral lesions and BMS.17,23
Five to 20% of the patients with pSS can develop peripheral nervous system disorders that complicate the symptomatology. The most common condition involving the cranial nerves is trigeminal neuropathy, which is normally bilateral and progressive, and is characterized by numbness or paresthesia, with or without pain. Sensory neuropathies are more frequent than motor dysfunction, which, if it develops, can affect the facial nerve.17,24
Dysphagia/dysgeusiaBecause of dry mouth, patients with pSS have difficulty chewing, speaking and swallowing food. To enable them to swallow food, they have to drink greater amounts of water. They also tend to tolerate highly flavorful foods poorly because of the altered taste sensitivity.6,17
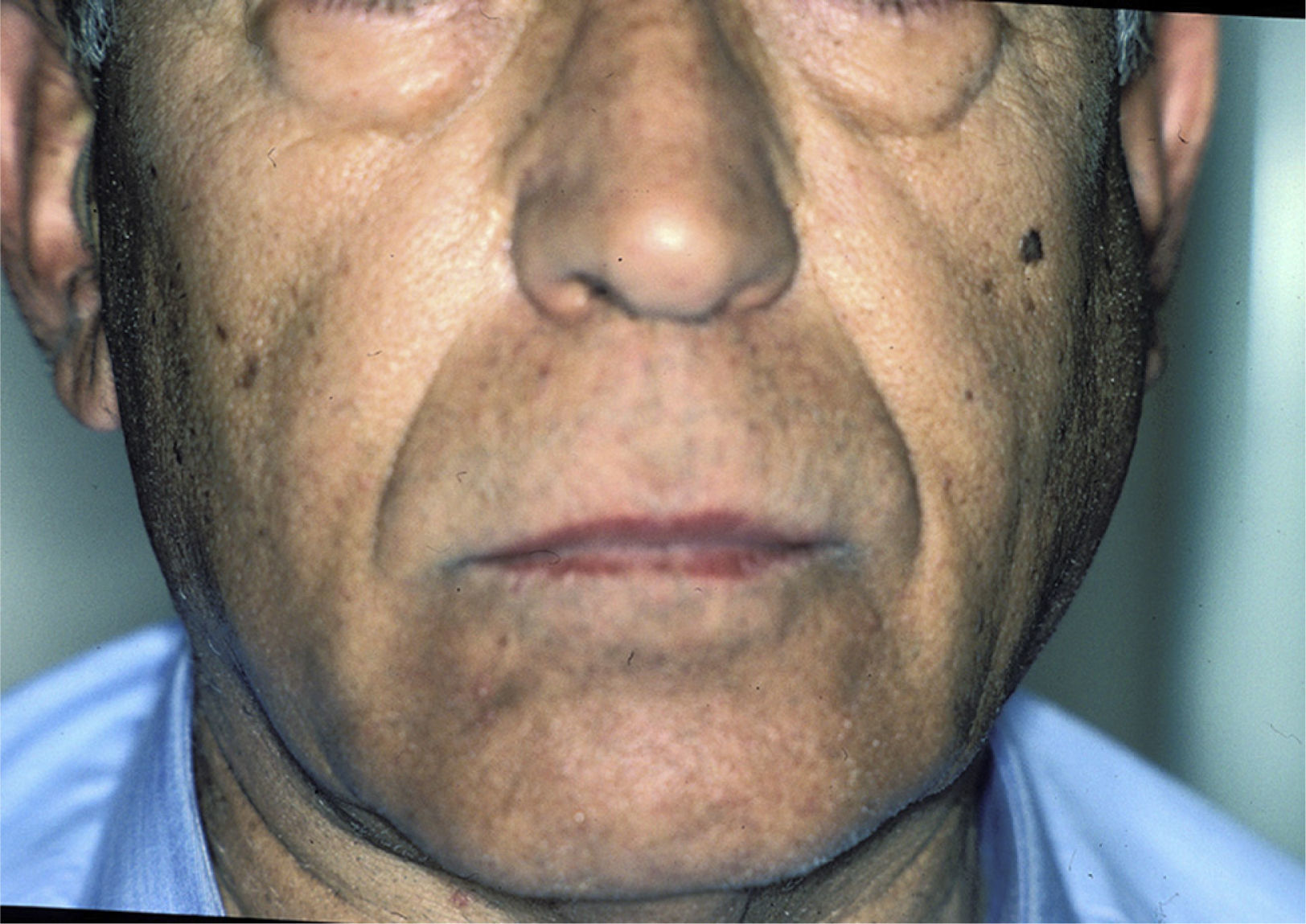
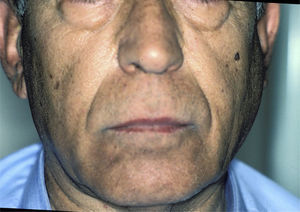
Salivary Gland InflammationThe enlargement of the salivary glands is characteristic of pSS (Fig. 5). The parotid gland is affected in 30%–40% of these patients. The inflammation of a single gland can be due, among other things, to an infection; however, if it persists over time, the possibility of a lymphoma must be considered.17
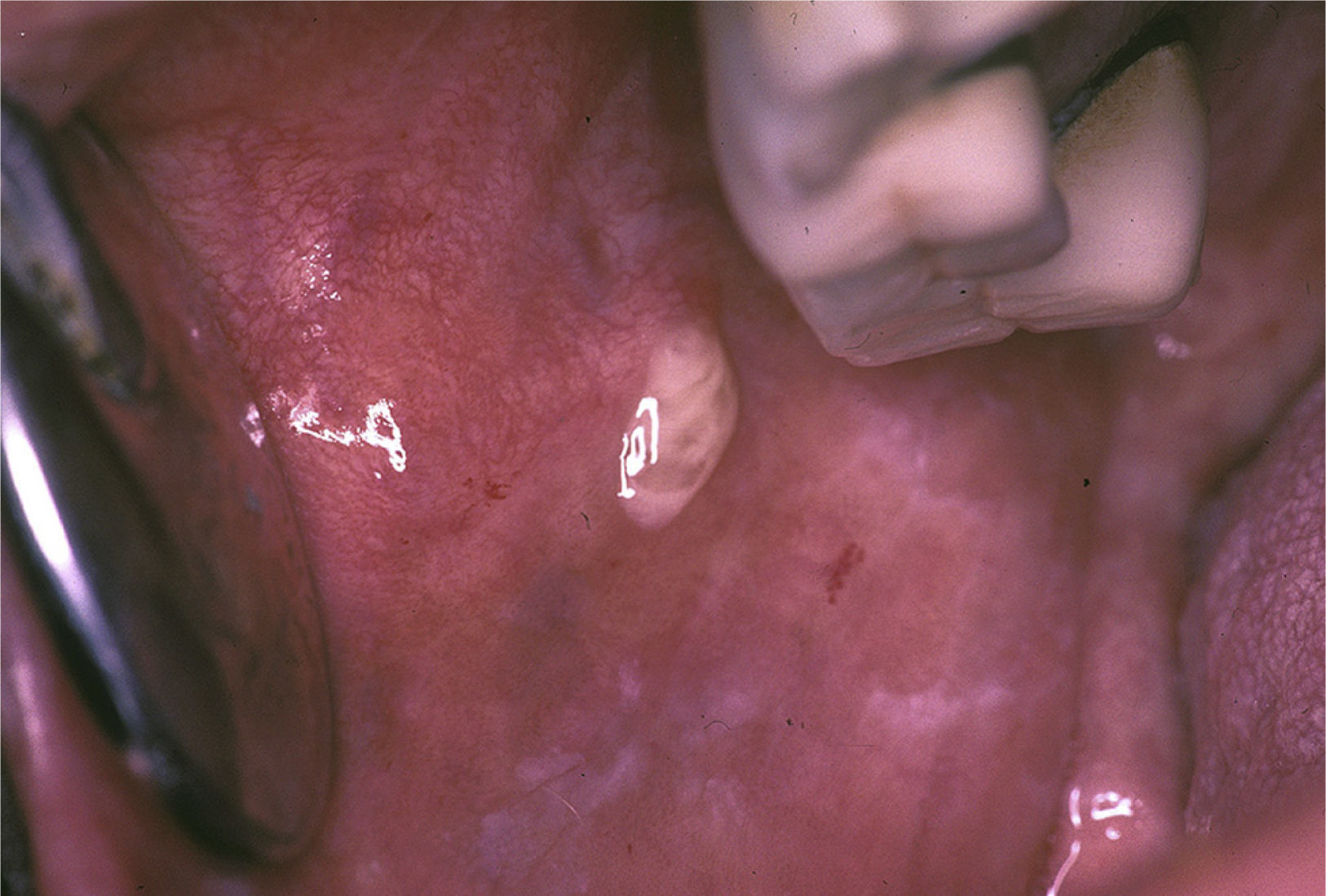
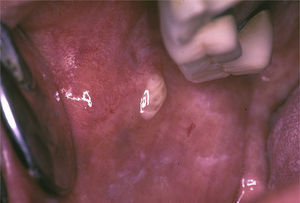
Bilateral swelling of the parotid glands can be found in 25%–60% of the patients with pSS, and can be acute or chronic. When acute, it can be bilateral or unilateral, it is usually accompanied by pain and is usually due to the obstruction of the salivary glands by a mucous plug, which is formed when the major part of the saliva is produced by the mucin-secreting cells. It will sometimes be necessary to eliminate the mucus by endoscopy and, in those cases in which more than one bacterial infection develops, in which pus usually appears in Stensen's duct (Fig. 6), antibiotic therapy appropriate for oral flora (gram-positive cocci) will be administered.25
The inflammation can affect the parotid and/or submandibular glands. The inflammatory infiltrate is mixed, with T and B cells and abundant cytokine secretion that promote local chemotaxis, exacerbating the gland inflammation. In these cases, the glands are painful on palpation, but pus is not expelled via the excretory duct.25
Gastroesophageal RefluxGastroesophageal reflux is a frequent finding in patients with pSS. The most common symptoms are acid indigestion, or heartburn, and regurgitation. The reflux can also produce oral lesions, especially dental erosion.17
Oral Lesions of Autoimmune EtiologyIn the medical literature, there are studies that demonstrate a certain association between pSS and the presence of oral lesions of autoimmune etiology, such as lichen planus, recurrent aphthous stomatitis, mucous membrane pemphigoid and pemphigus vulgaris.26
ComplicationsThe major orofacial complications in pSS are tooth loss, which makes it difficult for the patient to eat, and the development of lymphoma.
The high rate of caries in patients with pSS is associated with frequent tooth loss. Lost teeth can be replaced by removable prostheses or dental implants. Due to the dryness of the mouths of these patients, dentures are poorly retained, irritate the mucosa and are not well tolerated. There are dentures with reservoirs for artificial saliva, but the scientific literature on the subject reports contradictory results. There are few studies on treatment with dental implants and most involve small numbers of patients and case reports, but the results appear to be promising. Dental implant treatment in patients with pSS can be recommended to rehabilitate these individuals, as the disease does not affect bone health or osseointegration. Certain drugs that can affect the procedure, such as corticosteroids or bisphosphonates, should be taken into account. Thus, before undertaking implantation, dentists should consult with their patients’ rheumatologists.4,27,28
Patients with pSS are at higher risk of developing lymphoma than the general public, a difference that varies depending on the series (standardized incidence ratio [SIR] 2.52–48.1). Five to 10% of the patients will develop lymphoma over a 10-year period. The majority are B-cell lymphomas and, undoubtedly, the most common type is mucosa-associated lymphoid tissue (MALT) lymphoma, although paraproteinemia, light-chain gammopathy, T-cell lymphoma and Hodgkin's lymphoma have also been reported. MALT lymphoma is usually low-grade (stage I or II in 70%) and indolent, with low tumor mass; it is nodal in 15% of the cases, extranodal in 46%, and nodal and extranodal in 29%; lactate dehydrogenase and β2-microglobulin levels are usually normal; in 20% of the cases, it is detected at more than one extranodal site; it can affect regional lymph nodes; and there is bone marrow infiltration in 7%. The most common symptoms are: parotid swelling and the absence of B symptoms (fever, weight loss, night sweats). It is most often found in the salivary glands, especially the parotid glands; other reported sites are stomach, nasopharynx, skin, liver, kidney and lung. The transformation to lymphoproliferative malignancy is characterized by the early development of certain features: parotid enlargement, splenomegaly, lymphadenopathy, cutaneous vasculitis, hypocomplementemia, cryoglobulinemia, anemia, lymphopenia, CD4+ lymphopenia, neutropenia and anti-Ro/La antibodies. The detection of these predictive markers is crucial for the clinical follow-up and therapeutic intervention.29
Therapeutic Strategies in Dry MouthStrategies for Increasing Salivary FlowFor individuals with dry mouth, it is essential that the attempt be made to achieve adequate levels of saliva in order to eliminate the symptoms, prevent caries and infections, and enable them to speak, eat and swallow properly. For this purpose, there are different therapies that will be utilized in accordance with the degree of oral dryness. It is absolutely crucial that patients not be led to have false expectations, since it is not always possible to satisfy their needs. Below we suggest the use of different treatments depending on the severity of the hyposalivation, either by means of salivary stimulants (local or systemic) or using saliva substitutes (Table 5):
- •
Local salivary stimulants: saliva production can be stimulated with sugarless chewing gum, which raises the pH and buffering capacity, and sugarless hard candy, especially lemon-flavored, which stimulates the taste buds, thus favoring the output of saliva.4,6,11
- •
Other alternatives, much more complex and costly, have also been proposed, such as vibro-tactile stimulation of salivary output, which stimulates the residual secretory capacity through modulation of the autonomic reflex arc that regulates salivation; this is carried out by means of intraoral electrostimulation devices. At the present time, the scientific evidence is too limited to establish the effectiveness of electrostimulation in the treatment of xerostomia or hyposalivation in patients with pSS.7,30
- •
Systemic salivary stimulants: cholinergic drugs are used to treat xerostomia and xerophthalmia. Among them, that most widely studied and utilized is pilocarpine (at doses of 5mg/6h before meals and before bedtime, with a maximum of 30mg/day). It does not produce good results in all the patients and has contraindications and secondary effects that limit its use (Table 6). These drugs should be used in patients with residual salivary gland function. Studies show an improvement in oral dryness with these agents in up to 60%–70% of the patients.7,11,31–37
Table 6.Secondary Effects and Contraindications of Pilocarpine.
Secondary effects Profuse sweating Nausea-vomiting Sialorrhea Tearing Miosis Nystagmus Bradycardia/tachycardia/atrioventricular block/other arrhythmias Vasodilation/flushing Hypertension Polyuria Nervousness/seizures/tremor Bronchospasm Abdominal pain Constipation Flu-like syndrome Contraindications Uncontrolled bronchial asthma Chronic obstructive pulmonary disease Any eye disease in which the induction of miosis is contraindicated (iritis, angle closure glaucoma) Pregnancy/breastfeeding Allergic reaction to the drug - •
Saliva substitutes: there is a wide variety of products to reduce the sensation of dry mouth in patients with pSS. They all contain substances with an aqueous component supplemented with calcium, phosphate and fluoride ions. These products prevent demineralization and are available in mouthwash, gel and spray form and in intraoral devices. There are no conclusive studies on their efficacy in the prevention of dental caries.4,37,38
Therapeutic Strategies to Increase Salivary Flow According to Severity of Oral Dryness in Patients With Primary Sjögren's Syndrome.
| Grade | Characteristics | Therapeutic recommendations |
|---|---|---|
| 1 | Mild, sporadic discomfort on eating dry or course foodsNo signs of oral drynessUnstimulated salivary flow≥0.25ml/minStimulated salivary flow≥1ml/min | Education and modification of dietElimination, if possible, of xerostomia-related medicationAvoidance of alcohol and tobacco useHydration |
| 2 | Mild intermittent discomfort not only when eatingNo evidence of oral drynessUnstimulated salivary flow<0.25 and>0.1ml/minStimulated salivary flow<1 and >0.7ml/min | Add to grade 1:Use of sugarless chewing gum and sugarless lemon-flavored hard candyUse of saliva substitutes: gel and spray |
| 3 | Frequent constant discomfort not only when eatingDifficulty in eating, swallowing and speakingSigns of oral dryness can be observedUnstimulated salivary flow≤0.1ml/minStimulated salivary flow≤0.7ml/min | Add to grades 1 and 2:Use of artificial salivaIf there is no improvement with artificial saliva, use of systemic stimulants (after salivary gland biopsy to determine whether there is a certain degree of salivary function) |
| 4 | Permanent discomfortGreat difficulty in eating, swallowing and speakingAdvanced signs of oral drynessUnstimulated salivary flow≤0.05ml/minStimulated salivary flow≤0.3ml/min | Add to grades 1, 2 and 3:Consider the use of intraoral devices with artificial |
Patients with pSS should avoid the use of irritants such as alcohol and tobacco, and oral hygiene is essential. Water with sodium bicarbonate, tea and saline solutions should be used with caution since, in abundance, they eliminate the small amounts of mucous saliva from the oral tissues, a circumstance that can increase the sensation of dry mounth.7
Strategies to Prevent CariesIn the treatment of caries, it is essential to evaluate the oral status of the patients and the risk factors that favor the development of new caries. Patients with pSS must be involved in their oral care, applying proper oral hygiene practices, avoiding cariogenic foods and visiting their dentists as frequently as they are recommended to. A rheumatologist and a dentist should evaluate a patient's medical and dental records. Factors including diet (intake and frequency of the consumption of sugar-sweetened products), salivary flow, caries activity (number of new caries, for example, each year), hygiene measures and the use of specific products to prevent caries (fluoride) should be taken into account for the purpose of modifying the bad habits the patient may have.4,6
Oral hygiene depends on the skill of each individual. The dentist should instruct the patient on how to put into practice correct oral hygiene, as there are studies that show that careful oral hygiene with fluoride toothpaste is effective in controlling the development and progression of caries. However, the benefits of the use of fluoride in the control of dental caries are lost if they are not accompanied by good plaque control.
With respect to diet, it is necessary to know the total food intake. The patient should be asked to record all the foods and beverages consumed over one week to enable the dentist to indicate the modifications to be introduced. A high intake of sugar-sweetened products results in a greater number of caries; moreover, if the sugar-sweetened products are consumed between the main meals, the risk of dental caries increases even more. These patients are also recommended to avoid drinking beverages with a high sugar content (sucrose, maltose and lactose) and carbonated beverages.4
Calorie-free sweeteners (aspartame, acesulfame potassium, cyclamate and saccharine) do not produce caries, as they are not metabolized into acids. Sugar alcohols like sorbitol and xylitol have low cariogenic potential. Sorbitol is fermented by oral bacteria, but the acid production is low. Xylitol is not fermented by oral streptococci and, thus, does not lower the salivary pH; moreover, it appears to have a bacteriostatic effect.4,6
Chemical control of dental plaque is utilized to reduce the adhesion and growth of the microorganisms present in the plaque. These compounds are available in sprays, gels, varnishes, chewing gums, toothpastes and mouthwashes. Those most widely used are4:
- •
Chlorhexidine: a bacteriostatic agent that inhibits certain enzymes that interfere with bacterial accumulation. The combination of this compound with fluoride hinders the progression of dental caries.
- •
Xylitol: does not promote dental caries, reduces the number of bacteria and appears to inhibit glycolysis. It is present in many dental hygiene products.
- •
Fluoride: prevents dental caries. Its application in the dentist's office and at home, together with a controlled diet and dental hygiene measures, is highly important. It is found in gels, varnishes (normally applied in the dentist's office) and mouthwashes.
In addition to these 3 substances, new products are being studied. Probiotics interfere with the formation of the dental pellicle, disturb the ecosystem of dental plaque and produce substances that inhibit bacterial growth; in some cases, they also modulate or stimulate the patient's immune response. In dentistry, these substances are employed in the prevention of caries, periodontal disease and oral candidiasis, despite there being no conclusive results in this respect. Phosphoproteins/phosphopeptides serve to stabilize the calcium and phosphate of the teeth. They are present in natural substances such as saliva and milk, and there are studies that demonstrate that they inhibit demineralization and promote remineralization. Mouthwashes with these components have been utilized in patients with pSS, and have exhibited little efficacy in the control of caries.4
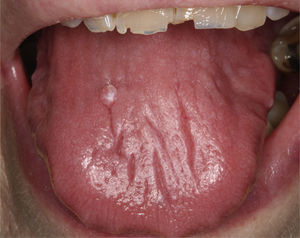
Rheumatologist–dentist RelationshipFor the correct diagnosis and management of the pSS patient, it is essential that there be a close relationship between the rheumatologist and the dentist. When a patient with xerostomia visits the dentist, the latter should pay special attention to the signs of dry mouth described above, rule out other conditions (the use of certain medications and other diseases) and quantify the stimulated and unstimulated salivary output. If hyposalivation is confirmed (Fig. 7) and the patient has no other diseases related to that condition, the dentist should consider the need for a biopsy of the minor salivary glands and refer the patient to the rheumatologist for completion of the study. The rheumatologist should refer any possible pSS patient to the dentist for a complete oral evaluation and the necessary supplemental studies.
The dentist will establish the recommendations in terms of hygiene, diet and oral treatment depending on the severity of the condition, and schedule appointments with the dental clinic, which, during the first year, should be at intervals of no more than 3 months; this will enable the dentist to assess the risk of new caries and the response to treatment. If the patient were to have grade 1 involvement (Table 5) and developed no caries during the first year, the appointments could be scheduled for every 6 months. The quantification of stimulated and unstimulated salivary output should be performed at least once a year and after any change in therapy, especially if the treatments are systemic. This will enable an objective evaluation of the patient.
Correct and smoothly flowing communication between the dentist and the rheumatologist is essential. Knowing all the changes observed in the patient and therapeutic modifications would enable both professionals to strengthen those aspects of the patient's behavior that could improve his or her oral status.
In conclusion, we feel that it is important that the patient with pSS receive a correct diagnosis of the oral manifestations, correct guidance on dietary and hygienic measures and correct management of hyposalivation, for the purpose of increasing the production of saliva and reducing the production of caries and oral infections. To achieve this, a good rheumatologist–dentist relationship is imperative and indispensable, and the outcome will be the correct treatment of these patients.
Conflict of InterestThe authors declare that they have no conflicts of interest.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Please cite this article as: López-Pintor RM, Fernández Castro M, Hernández G. Afectación oral en el paciente con síndrome de Sjögren primario. Manejo multidisciplinar entre odontólogos y reumatólogos. Reumatol Clin. 2015;11:387–394.