Bone tissue is a highly regulated structure, which plays an essential role in various physiological functions. Through autocrine and paracrine mechanisms, bone tissue is involved in hematopoiesis, influencing the fate of hematopoietic stem cells. There are a number of molecules shared by bone cells and immune system cells indicating that there are multiple connections between the immune system and bone tissue. In order to pool all the knowledge concerning both systems, a new discipline known under the term “osteoimmunology” has been developed. Their progress in recent years has been exponential and allowed us to connect and increase our knowledge in areas not seemingly related such as rheumatoid erosion, postmenopausal osteoporosis, bone metastases or periodontal disease. In this review, we have tried to summarize the most important advances that have occurred in the last decade, especially in those areas of interest related to rheumatology.
El tejido óseo es una estructura fuertemente regulada, que desempeña un papel esencial en diferentes funciones fisiológicas. A través de acciones autocrinas y paracrinas participa en la hematopoyesis, influyendo en el destino de las células madre hematopoyéticas. Existe además una serie de moléculas compartidas y también múltiples conexiones entre el sistema inmune y el tejido óseo. Con el objetivo de agrupar los conocimientos que relacionan ambos sistemas, se ha desarrollado una nueva disciplina conocida con el término «osteoinmunología». Su progresión en los últimos años ha sido exponencial y nos ha permitido relacionar e incrementar el conocimiento en temas aparentemente alejados como la erosión reumatoide, la osteoporosis posmenopáusica, las metástasis óseas o la enfermedad periodontal. En la presente revisión hemos tratado de resumir los avances más relevantes que se han producido en la última década, sobre todo en aquellos aspectos que interesan de manera preferente a la reumatología.
The adult human skeleton is composed of 213 bones, excluding sesamoids, and constitutes 20% of body mass.1 It does multiple (Table 1) and, up to a certain point, antagonistic functions, as it needs to provide strength and resistance to adequately protect the vital organs it hosts and, simultaneously, it has be light enough to allow movement without too much muscle effort.2 In addition, bone tissue is the source of great secretory activity, participating both in local and distant processes, through the production of several hormone-like proteins that intervene in processes such as calcium homeostasis, renal function or energy metabolism.
Properties and Functions of Bone Tissue.
| Properties of bone tissue | Main functions |
| Strength | Structure support |
| Resistance | Load support |
| Propulsion against gravity | |
| Lightness | Locomotion |
| Flexibility | Energy absorption/recuperation |
| Auto-repair capacity | Fracture healing |
| Fatigue and microdamage management | |
| Active metabolism | Calcium/other ion homeostasis |
| Growth factor and cytokine reservoir | |
| Endocrine action (renal function, energy metabolism) | |
| Hematopoiesis |
Considered for a long time a static structure, bone behaves, as a matter of fact, as a very active organ, constantly related to other organs and systems, with permanent cell activity. In order to successfully accomplish the abovementioned functions, the skeleton uses a unique biological system called bone remodeling (BR), through which it renews itself totally every 10 years throughout adult life. This process, which begins before birth, is crucial in adolescence, when bone formation exceeds resorption, allowing for the acquisition of 40% of total bone mass and reaching peak bone mass.3 In the following years it reaches equilibrium and a stable bone mass until, some years before menopause in women and at older ages in men, BR is inverted resulting in a predominance of resorption over formation, which leads to the gradual reduction of the bone mass achieved at the end of bone maturation. This acceleration of remodeling, when excessive, is the main cause of osteoporosis.4
BR is a necessary process and is tightly controlled, with mechanical factors and a delicate cortege of cells and molecules, originating from the local bone marrow and regions distant to bone itself, intervening in it. Physiopathological basis has been established during the past 10 years that has allowed us to increase our knowledge of the mechanisms controlling the relationship between the cells and molecules of the immune response and bone cells. We have learned that these complex interactions are fundamental in understanding the mechanism characterizing diseases as different in their expression as arthritis, osteoporosis or cancer. With the intention of grouping all of this knowledge, a new scientific discipline has been developed, named “osteoimmunology”, a term first used by Arron and Choi in 2000.5
Osteoimmunology studies the relationship between the immune system and the skeleton (osteoimmune system), analyzing its interdependence at the anatomical, vascular, cellular and molecular levels, with a special emphasis on the development of knowledge of ligands, receptors and intracellular signaling molecules governing these processes. Its clinical interest field is ample and is especially essential in processes such as arthritis, inflammatory bowel disease, osteoporosis, cancer and periodontal disease, among other.6 This review aims to present the most relevant progress achieved in the field during the past decade, especially those aspects that affect rheumatology specifically.
Anatomical and Functional Relationships Between the Immune System and BoneThere are multiple anatomical and vascular contacts, as well as different cellular and molecular mechanisms allowing a permanent interaction between bone and the immune system in such a way that it is possible to consider them as integrated functional unit7 (osteoimmune system). Bone, by virtue of its structure, is related interiorly with the bone marrow, participating in hematopoiesis and allowing the local cooperation of bone and immune cells, both locally and at a distance through nutritious and periosteal vasculature while, through enthesis and the juxtaarticular skeleton, connecting with structures which are essential in the joint destruction that characterizes chronic inflammatory joint disease.
Cells of both systems share a common origin; osteoclasts (OC) come from hematopoietic stem cells and share their lineage with monocytes and macrophages while mesenchymal stem cells are the precursors of osteoblasts (OB), while, in turn, play a central role in the differentiation of hematopoietic stem cells in the spaces adjacent to the endosteal surface.8 Some molecular pathways that participate in remodeling, such as parathryroid hormone (PTH), bone morphogenetic protein and the Wnt pathways, also participate in the regulation of hematopoiesis. On the other hand, multiple cytokines from lymphocytes, dendritic cells and macrophages act on BR cells, both in its resorptive phase, generally through the RANK/RANKL/OPG pathways, as in the bone formation phase, directly or indirectly through Wnt signalling.9
One of the most relevant unresolved questions is the role of the immune system in the development of normal skeleton. From an ontogenic point of view, the skeleton precedes the development of the immune system, making intervention unlikely, at least in early phases of formation and acquisition of bone tissue functions. However, it is well known that modeling and remodeling occur during all the adult life and that are produced in concrete spaces sharing a certain similarity with the closed compartments where hematopoiesis develops, another process that is active until death. The marrow spaces, called “stem cell niches” (SCN), whose structural support and maintenance is strongly dependent on bone cells and are necessary for the development of the immune system and allow the interaction of immune and bone cells, bidirectionally.
Bone homeostasis, greatly dependent on remodeling sites, is regulated by immune responses, especially if they are due to pathological situations. With aging an accumulation of memory T lymphocytes in the bone marrow, activated throughout a life of continuous exposure to antigen, expressing RANKL on their surface and may facilitate the activation of BR that is seen in certain situations associates to aging.
Bone RemodelingBR is produced in specific sites known as bone remodeling compartments, composed of a functional cohort of cells that act in a coordinated way, called basic multicellular unit and which contains OC, OB, osteocytes (OS), bone lining cells and support capillary cells.10 The cycle is structures in 4 consecutive phases, not symmetrical or of the same duration though, starting with the recruitment of OC precursors to the quiescent surfaces, on the trabecular or endosteal surfaces or in the depths of an osteon. Mature OC activate their resorptive machinery and carve a lagoon (Howship's lagoon) that, in the next phase (inversion phase) is leveled out by the collaboration of macrophage lineage cells of the lining, letting mature OB fill the cavity with osteoid. Finally, mineralization and mature bone formation occurs.
In the absence of disease, the difference between the short resorption period and the long formation one (remodeling space) does not lead to relevant structural consequences. However, when disequilibrium occurs between both processes and the frequency of remodeling unit activation is increased, as occurs in menopause, the remodeling space reaches considerable dimensions and may produce an increase in bone fragility (many open lagoons without the conclusion of osteoblast filling).
In the normal adult skeleton there are about 2 million remodeling sites that work at a speed of 25μm per day and replace a bone volume of 0.025mm3 in each microsite. The interval between cycles in the same localization varies from 2 to 5 years and the rate of remodeling of the skeleton as a whole is 10% annually (3% cortical bone and 30% trabecular bone). The half life of the OC is 2 weeks and their final destination, under normal conditions is apoptosis, while the half life of active OB is 3 months and their fate is threefold as they may suffer apoptosis or be inactivated as lining cells or buried in the depths of the mineralized bone matrix which they formed themselves, transforming into OS.11
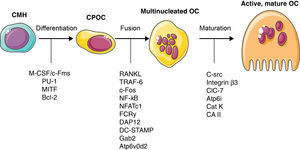
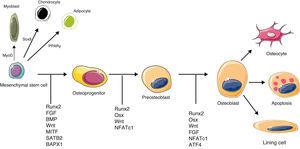
Bone CellsOsteoclastsOC comprise only 1%–2% of bone cells. Their morphology is characteristic when they are activated and are easily recognized in non-decalcified samples as strongly polarized multinucleated structures, with a basal external signal exchange region and a calcified bone bound region, possessing a specific structure called brush border. They belong to the monocytic-macrophage lineage (Fig. 1), although, in contrast with other members of this family, in their mature stage they are capable of binding to bone through integrins αvβ3 expressed on the surface of the podosomes and have the property of interacting with matrix proteins such as osteopontin and vitronectin. After the primary activation signal an actin ring is produced and the zone is hermetically sealed, allowing the exchange of ions and proteases necessary for bone resorption.
In the osteoclastic cytoplasm, carbonic anhydrase II dissociates cytosolic carbonic acid into protons (H+) and bicarbonate (HCO3−), the latter being exchanged for chloride (Cl−) though a specific channel, allowing for the conservation of the isoelectric intracellular state. The proton locates on the brush border where it is uptake by a proton pump dependent on a specific ATPase (H+-ATPase), which transports it to the osteoclastic lagoon. In the vicinity of this pump an ionic channel can be found: chloride 7 or ClC7. In concrete, this channel interchanges 2 Cl for one H+ and is fundamental in the lysosomic acidification process in general and in bone resorption in particular.12 Its functional loss is one of the most common causes of osteopetrosis13 and constitutes, along with the proton pump, an interesting therapeutic target14 limited, for the time being, by its extraskeletal actions derived, especially, from the risk of production of lysosomal diseases.15
In the osteoclastic lagoon, through the binding of these 2 ions, chlorhydric acid is formed which acidifies the medium and leads to the dissolution of hydroxyapatite and the liberation of calcium and phosphorus, at the same time maintaining the equilibrium of the cytoplasmic ionic charge. Lastly, through lysosomes, a cystein protease is secreted, catepsin K, as well as a series of metalloproteases which, finally, lead to the dissolution of organic matrix. The resulting byproducts of degradation enter the OC through endocytosis and are transported to the basolateral region in tartrate resistant acid phosphatase rich vesicles and released to the exterior through exocytosis.
A series of signaling molecules participating in osteoclast activation and differentiation are currently known,16 summarized in Fig. 1. The initial intervention of M-CSF is necessary for its differentiation. During a second phase the expression of RANK on the membrane definitely characterizes the population that is destined to differentiate to OC. When this receptor binds its ligand (RANKL), the precursor cell commences maturation, becoming a multinucleated, polarized cell, capable of activating all of its resorptive machinery. The activation of RANK unleashes an intracellular signal through the TRAF6 adaptor factor and though several parallel signal cascades in which, finally the activation of NF-κB, c-Fos, phospholipase C and NFATc1 takes place.
Although RANKL's classical origin is situated on the OB, recent papers have shown that it is really the OS who provide a larger amount of this cytokine, which, on the other hand, is more logical due to the known role these cells have in the detection both of mechanical as well as hormonal signals, indicating that the OS could actually be the true regulators of BR, at least under physiological conditions. T and B-lymphocytes also produce RANKL, although their role is very likely only relevant in pathological conditions. Under physiological conditions, IFN-γ secreted by T lymphocytes inactivates TRAF6, the main adaptor protein for the RANK intracellular signal, constituting a particularly relevant regulating factor to impede osteoclast over activation by lymphocyte RANKL. This finding is also important due to its historical dimension; the publication of experimental evidence of this mechanism led to an accompanying editorial in which Arron and Choi used the term osteoimmunology for the first time.5 There is, in addition, a series of co-stimulatory pathways, such as the one used by the osteoclast-associated receptor17 and the triggering receptor expressed in myeloid cells-2,18 whose ligands and activation controls are unknown, but which could be necessary in inflammatory states, although their analysis exceeds the scope of this review.
OsteoblastsBone formation is dependent on the recruitment of a sufficient number of OB to the bone surfaces subjected to attack by OC. They originate from a subgroup of mesenchimal stem cells with an osteogenic differentiation capacity. Although the exact in vivo localization of these cells is unknown, recent studies suggest that they may be situated on the external surface of the medullary sinusoids and would reach the bone surface through vascular canals in response to signals coming from the remodeling sites, be it the resorpted matrix or the activated OC themselves. Once situated on the bone surface, OB produce organic matrix (osteoid) and finally undergo apoptosis or are “buried” in the calcified matrix, transforming into OS.19
Runx2 is the master regulator of osteoblastic differentiation, a marker that is prematurely expressed in this cell lineage.20 Although necessary, it is not enough for a progenitor cell to differentiate (Fig. 2). It is also important for endochondral as well as intramembranous ossification to occur, because Rnx2 null mice do not form mineralized bone in any region of the skeleton and lack OB as well as alkaline phosphatase, osteopontin and osteocalcin. Other factors intervene in a latter stage of differentiation, although the most relevant are PTH/PTHrp, GH/IGF-1 (which plays an important role in the maintenance of muscle and bone mass, and during aging), the BMP21 family (which belong to the TGF-β superfamily and are the most potent currently known osteogenic factors) and the Wnt pathway (see below). BMP2 and BMP7 are used clinically, at a local level, for spinal fusion and in binding defects of long bones, respectively.22
Molecular signals with a key role in osteoblast differentiation and activation. MyoD: myogenic differentiation 1 protein; PPARγ: peroxisome proliferator-activated receptor gamma; Sox9: sex determining region Y-box 9. Rest of abbreviations: see Term glossary in Annex 1.
OS are the most abundant cells (90%–95%) and the most long lived, reaching up to 25-year survival rates.23 Each OS has an elevated number (up to 50) of dendritic like cytoplasm prolongations that are distributed through the surrounding tissue and reach the surface, using canaliculi through which small molecules, such as nitric oxide and prostaglandins, circulate, which participate in an extensive signaling network that is being recognized as a fundamental part of the control of remodelling. These dendrites serve to bind to other, nearby OS and also to endothelial cells and superficial OC, for which specialized nodal structures exist24 containing integrins and connexins (mainly connexin 43).
It is obvious that the osteocyte–canalicular network works as a syncytium ideally situated and distributed to detect mechanical changes or deep bone lesions and establishes a communication system to attract the cells necessary to adapt the bone form to load (modeling) or start a new remodeling unit that repairs damage, provides new bone to the organism or responds to the systemic hormonal needs such as calcium homeostasis or renal function.25
The OS secretory activity is very intense, with a large number of molecules detected in the canaliculi, among which RANKL and OPG (signaling the activation of a bone remodeling site in response to mechanical stimuli), ATP (intracellular Ca2+ modulation), PGE2 (promoting osteoformation), nitric oxide (promoting osteoformation and inhibiting resorption), FGF23 (modulating cell proliferation and differentiation, with hormone-like systemic actions) and DMP-1 (which inhibits bone mineralization)23 stand out.
The role of osteocytic RANKL in osteoclastic activation has been recently manifested In addition and the Wnt/β-catenin pathway has an important role in osteoformation, constituting an attractive therapeutic target. This pathway, also known as the canonical Wnt pathway, is fundamental for the normal development of bone and cartilage. It also possesses an relevant, albeit poorly characterized, role in the adult skeleton, essential for BR.26,27
Wnt is a family of natural ligands (there are currently 19 different types) that bind cell membrane receptors and unleash intracellular actions that lead to the release of β-catenin and its translocation to the cell nucleus. The membrane receptor consists of a dual complex formed by Lrp 5/6 and frizzled, a 7 transmembrane domain receptor whose tridimensional structure has been recently characterized.28 When the Wnt ligand binds to this structure it produces a signaling cascade that leads to the inhibition of cytoplasmic GSK3 β, which releases β-catenin, a key mediator in the canonical Wnt pathway that, in the absence of this signal, remains anchored in a structure that leads to its proteosomal destruction and avoids its accumulation. When β-catenin reaches the nucleus it interacts with members of the TCF/Lef transcription factor family and contributes to the synthesis of proteins mainly involved in bone formation. The OS intervene in this pathway through the secretion of sclerostatin, a potent inhibitor of Wnt, which constitutes an attractive therapeutic target.
Immune Cells and Bone PathophysiologyThe enormous progress in the knowledge of the functions of RANKL and its receptors, as seen in the past decade, has been the decisive factor for the development of osteoimmunology. RANKL is essential for OC differentiation, activation, polarization and survival under physiological conditions. Its role in disease is characterized by accelerated bone destruction, such as osteoporosis, arthritis or bone metastasis is also very important, although some peculiarities with great clinical relevance have been noticed.
In murine arthritis models, RANKL or c-fms (both being lineages that lack OC because they do not respond to RANK or M_CSF respectively) deficient mice do not undergo bone destruction, even when bred with TNF transgenic mice, which develop spontaneous erosive arthritis. However, a similar degree of inflammation is seen, indicating that OC and RANKL are necessary for bone destruction but not for inflammation, which is regulated by different pathogenic pathways.29,30 This experimental evidence has been corroborated clinically by the observation that antiosteoclast treatment reduces bone loss but not inflammation.
When the Tnfsf11 gene, which codifies RANKL, is silenced, in addition to developing osteopetrosis due to a lack of OC, alteration in the development of lymphocytes and lymph node organogenesis may be seen. The directed deletion of other molecules may also produce effects on both systems. For example, mice lacking PU.1, a transcription factor regulating B cell function show a lack of differentiation of macrophages and the inhibition of osteoclastogenesis.31 The deficiency of transmembrane signal adaptors with ITAM motifs, such as DAP12, or of FcR-γ, leads to deficits in the activation of OC, and when the defects implies both molecules, severe osteopetrosis may be seen.32
There are, in addition, a series of co-stimulation molecules, whose role is mostly unknown, but could be important especially in pathologic states with osteoclastic activation. One of the molecules, called osteoclast-associated receptor, an IgG-like receptor whose ligand is unknown, transmitting an intracellular signal that complements RANK, through NFATc1, also modulating the activation and maturation of macrophage-monocyte lineage cells. This receptor may be relevant in the pathogenesis and severity of osteoimmune diseases and the identification of its (still unknown) ligand is a field of enormous interest.17 In summary, there is multiple experimental evidence that indicates that the skeleton and the immune system use the same signaling systems.
T cell infiltrate is one of the main characteristics of the rheumatoid synovial, although its role in osteoclastogenesis (and therefore in juxtaarticular erosion) has been a motive of great controversy. These cells, when activated, not only express RANKL, but also secrete, according to their profile, other cytokines such as IFN-γ in the case of Th1 lympohocytes,33 which possess an antiosteoclastogenic action, even at low concentrations, through the degradation of TRAF6 mediated through the ubiquitin–proteasome system.34 In addition, the expression of IFN-γ in synovial rheumatoid is low which, along with the above-mentioned, makes Th1 cells a very unlikely candidate in OC activation under this scenario.
Takayanagi et al.,35 pioneering osteoimmunology, have shown the relevance of the Th17 subpopulation as one of the main regulators of local osteoclastic activation in rheumatoid arthritis (RA). These cells, which may chronologically be defined as the third effector arm (after the initial discovery of Th1 and Th2) of T helper CD4+ cell population, are very relevant in the defense vs bacterial and fungal infections, but a loss of equilibrium may promote the appearance of autoimmune disease. Their development is produced in response to IL-6 and TGF-β, while IL-23 promotes its expansion. The transgenic deficiency of IL-17 in murine models or the therapeutic blockade using antibodies markedly reduces the development of RA, suggesting a very important pathogenic role for these cells, something we will analyze further.
Role of the Immune System in Postmenopausal OsteoporosisBone cells possess estrogenic receptors and it has been seen that these hormones may act directly on the skeleton through poorly defined protector functions, which could be a determinant in the pathogenesis of postmenopausal (PM) osteoporosis. On top of this action, estrogen indirectly acts regulating the production of cytokines by the bone cells themselves and by Y and B lymphocytes also, which will finally interact, leading to changes in the remodeling profile. A decade ago it was seen that an increase in the levels of TNF-α in the skeletal micromillieu due to a T cell expansion associated with an estrogenic deficit led to the thesis of this cytokine as the main actor in menopausal related bone loss.36,37 However, TNF-α mice model studies showed contradictory results38,39 and placed interest on the search for other protagonist cytokines in the accelerated resorption that characterizes the PM period.
There is evidence that shows an overregulation of RANKL after oophorectomy, both in PM bone marrow cells as in synovial cells in a murine experimental arthritis model.40–42 RANKL expression in recently menopausal women is threefold when compared to mononuclear bone marrow cells, both in preosteoblasts as in T and B lymphocytes, when compared to premenopausal women or those postmenopausal patients treated with estrogen. On the other hand, Yoneda et al. have provided additional proof of the relevant effect of RANKL by observing a marked increase of this cytokine in synovial cells of mice with an experimental model of RA.43
Estrogen also has an action on the osteoclast precursor and mature OC cell membrane RANK action, although the results of this receptors’ expression, in situation of hormonal deficit, have been contradictory. There is proof that estrogen intervenes in the RANKL–RANK intracellular signaling through recently revealed mechanisms. Through experiments done on murine monocytes it has been seen that these hormones block the AP-1 dependent transcription by suppressing c-Jun expression and its kinase mediated phosphorylation. Estrogens also inhibit the degradation of IkB, impeding the nuclear localization of NF-κB in OC, probably through the non-genomic effects that promote TRAF6 sequestration.44–46
In summary, there is abundant proof that confirms the potent antiressortive action of estrogen and that, part of these actions, is related to the RANKL/OPG/RANK system. However, it is still unclear if these actions are direct or indirect, implying other cytokines. It is an accepted fact that the production of RANKL by osteoblastic cells is stimulated in the presence of TNF-α, IL-1b, IL-11 and PGE247 and inhibited by TGF-β.48 In addition, the administration of etanercept or anakinra to women who have recently entered menopause reduces resorption markers in 50%, while different mice models has confirmed the relevant role these cytokines play in oophorectomy induced osteopenia.49 The function of other cytokines, such as IL-6 o IL-7, has not been sufficiently cleared, although it is likely that they also have a certain proresorptive role.
Bone Damage Mechanism in Rheumatoid ArthritisThe synovial membrane, composed by a mixed population of cells with fibroblast and macrophage phenotypic characteristics, is the site where the joint inflammatory process begins. This phenomenon is produced after the synovial infiltration by T and B lymphocytes, endothelial cells and activated macrophages, and leads to marked synovial hyperplasia and the destruction of bone located on the osteosynovial contact zones, leading to erosion and the invasion of adjacent marrow spaces by the neoformed inflammatory tissue.50
In the same manner, two other forms of bone loss are known in RA: periarticular osteopenia that affects trabecular and cortical bone in the vicinity of the inflammatory process, and generalized osteopenia produced in regions distant to the joint tissue.51 In both cases there is a reduction in bone mass, albeit due to different mechanisms. In systemic osteopenia, remodeling is accelerated although remains couples, with a negative final balance, while in periarticular bone loss there is a primary osteoforming deficit, probably associated with a lack of balance in coupling mediated by, among others, Dkk-1. In this review we will concentrate on the focal lesion that leads to erosion as a primordial characteristic of the disease.
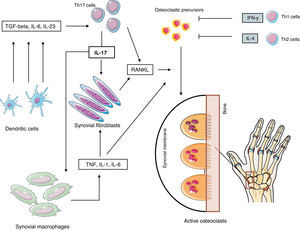
Erosion effector cells, situated in the contact zone of synovial pannus and marginal bone, express catepsin K, tartrate resistant acid phosphatase, β3 integrin and calcitonin receptor mRNA, phenotypic markers characteristic of mature OC. In rheumatoid synovium there are multiple cytokines with osteoclastogenic activity (Table 2), RANKL, M-CSF, IL-1, IL-17 and TNF, among others, although the mechanisms leading to erosion are still poorly defined. It is a know fact that synovial infiltration by T cells is one of the main characteristics of RA and that these cells, once activated, express RANKL, making them candidates for osteoclastic activation. However, these cells secrete other cytokines such as IFN-γ (Th1) and IL-4 (Th2), which have a potent inhibiting effect on osteoclastic differentiation of monocyte-macrophage lineage cells.52
Cytokines and Local Factors Potentially Involved in Rheumatoid Erosions.
| Cytokine | Originating cell population in RA | Inflammatory effect | Osteoactive effect |
| TNF-α | Macrophages and dendritic cells | Main proinflammatory CK in RA | Indirect osteoclastic activation through RANKL. Direct probable action through TNF 1 receptor. Inhibits osteoformation |
| TGF-β | Treg and dendritic cells | No | Indirect osteoclast activationInhibits osteoblast differentiation |
| GM-CSF | Th1 cells | Yes. Probable role in local activation and recruitment of macrophages and PMN | Inhibits osteoclastogenesis |
| IFN-γ | Th1 cellsNK cells | Yes | Inhibits osteoclastogenesis |
| IL-1 | Macrophages and dendritic cells | Yes | Potent stimulator of osteoclastogenensis and activation |
| IL-2 | Th1 cells | Yes | |
| M-CSF/IL-3 | Th1 and Th2 cells | Yes | Inhibits osteoclastogenesis |
| IL-4 | Th2 cellsMastocytes | Pro and antiinflammatory | Inhibits osteoclastogenesis |
| IL-6 | Macrophages, dendritic cells and synovial fibroblasts | Main proinflammatory CK | Indirect osteoclastic activation |
| RANKL | Synovial fibroblasts, osteoblasts and Th17 cells | No | Direct osteoclast activation through RANK |
| IL-10 | Macrophages, Th2 cells, Treg cells | Antiinflammatory action | Antiosteoclastic action |
| IL-15 | Monocytes, macrophages, dendritic cells and fibroblasts | Proinflammatory action | Indirect osteoclastic activation |
| IL-17A | Th17 cells, Tуδ cells, mastocytes | Main Th17 effector cytokine | Indirect osteoclast activation through RANKL and through the inhibition of IL-4, IFN-γ and IL-12 |
| IL-17F | Monocytes, Th17 cells, monocytes | Weak inflammation mediator through Th17 | Probable osteoclastogenic action |
| IL-21 | Th17 and NK cells | Yes, through Th17 autocrine amplification | Indirect RANKL dependent osteoclast indirect activation |
| IL-22 | Th17 cells, NK cells, dendritic cells | Yes, through TNF and IL-6 induction | Indirect osteoclast activation |
| IL-23 | Dendritic cells and macrophages | Yes, through Th17 | Indirect osteoclast activation |
| IL-27 | Dendritic cells and macrophages | Dual function, dependent on cytokine micromillieu | Inhibits osteoclast precursor formation, blocking RANK dependent intracellular signals |
| IL-32 | NK cells, synovial fibroblasts | Yes. Predominantly intracellular functions. Mutual TNF-α dependency. | Promotes, but does not directly activate, osteoclast activity |
| IL-33 | Synovial fibroblasts | Undefined mixed actions | Osteoclastogenensis inhibition |
| Dkk-1 | Osteocytes and osteoblasts | Global and soluble inhibitor of the Wnt pathway. No clear inflammatory actions in RA | Key osteoformation inhibitor in RA |
| sFRP | Fibroblasts and synovial macrophages | Wnt soluble inhibitor with no effect on inflammation in RA | Osteoformation inhibitor |
| Sclerostin | Osteocytes, chondrocytes | Inhibitor of Wnt pathway, with more selective functions than Dkk-1 | Osteoformation inhibitor |
RA: rheumatoid arthritis; CK: cytokine; Dkk-1: Dickkopf-1 protein; GM-CSF: granulocyte–monocyte colony stimulating factor; IFN-γ: gamma interferon; IL: interleukin; M-CSF: macrophage colony stimulating factor; NK: natural killer; PMN: polymorphonuclear; RANK/RANKL: NF-κB/RANK ligand receptor activator factor; sFRP: secreted frizzled-related protein; TGF-β: transforming growth factor beta; TNF: tumor necrosis factor; Treg: regulator T cells; Wnt: Wnt signaling pathway, a term derived from the name of the Wg (wingless) gene of Drosophila and its mammalian homologue Int (Integration 1).
Other experimental findings have added negative evidence on the role of T cells, both Th1 and Th2, as direct effectors of osteoclast activity as well as in the production of erosions. For example, co-cultures of activated T cells with OC precursors show a predominantly inhibiting effect on the latter, while in murine models of collagen induced arthritis, the severity of local bone damage could be increases in the absence of IFN-γ or IL-4 signaling. All of these data suggest that both Th1 and Th2 cells do not directly intervene in local bone destruction.53,54
Th17 cells, considered the third effector arm of activated T CD4+ cells,55 have a series of characteristics that implicate them directly in local osteoclastic activation in RA: they do not produce significant amounts of IFN-γ, they provoke local inflammation and cytokines that induce the expression of RANKL on synovial fibroblasts simultaneously while themselves expressing RANKL with a capacity of directly activating osteoclastic precursors. Their differentiation is unleashed by the combination of IL-6 and TGF-β, while IL-23 is required for their development and effector functions, but is not obligatory for the inset of differentiation of its naïve precursor.
Mature Th17 cells produce IL-17, IL-21 and IL-22, cytokines that have a proinflammatory effect and can currently be considered the longed for osteoclastogenic Th subtype. Therefore, these cells produce IL-17 which induce the expression of RANKL in synovial fibroblasts that directly contact RANK on precursors, leading to the differentiation of OC which then migrate to the marginal zone, initiating bone erosion, In addition, IL-17 increase local inflammation through the production of inflammatory cytokines such as TNF, IL-1 and IL-6. We may conclude that Th17 cells have the capacity to produce both inflammation local bone destruction and are an interesting therapeutic target, currently under study due to its clinical development potential56–61 (Fig. 3).
Pathogenesis of rheumatoid erosions. Abbreviations: see Term glossary in Annex 1.
One of the characteristics of rheumatoid erosions is the notable absence of perilesional healing, opposed to what is seen in other inflammatory diseases such as psoriatic arthritis or ankylosing spondilytis.62 This has traditionally been related to local inflammatory activity that would slow osteoblastic work in the proximity of the erosion.63 However, the use of potent anti-inflammatory drugs, such as TNF or IL-6 inhibitors, although slowing the progression of the erosion, only leads to slight signs of repair, in the form of new bone deposition, adjacent to the juxtaarticular bone marrow.64–67
Mouse models have observed that the population of osteoblast precursors expressing Runx2 is elevated in swollen areas, with a low expression of alkaline phosphatase, indicating active mineralization.68 These immature cells could produce osteoid but not mineralize it, conserving therefore the normal expression of RANKL and, therefore, their capacity to activate OC.
As a whole, the results seen suggest that the inflammatory process in RA produces a disequilibrium between bone resorption and formation, stopping the formation of mature OB, but not the influx of Runx2 positive precursors. These cells have a phenotype that recalls that seen on mice with deficient β-catenin, something that has made the Wnt pathway interesting as a possible dysfunctional pathway involved in the lack of repair of the rheumatoid erosion.
Several families of endogenous inhibitors of the Wnt pathway are currently known, mainly due to their extraordinarily relevant role in multiple development and activation functions on different cell systems, and which are tightly regulated. Among others, sclerostin, Dkk-1 and sFRP, are the best characterized.69,70 Elevated serum levels of these molecules are seen in RA and treatment with a neutralizing monoclonal antibody to Dkk-1 improves local erosion in the hTNF.Tg RA mouse, inverting the “astenic” pattern of the typical erosion, making it a proliferative erosion. One very interesting hypothesis to consider is that these inhibitors would be responsible of the lack of considerable repair seen in the rheumatoid erosion, but the specific role of each, their origin and the consequences of its inhibition are still poorly characterized aspects that should be further studied due top their important pathogenic and therapeutic potential.
RANKL Immunomodulating FunctionsThe role of RANKL in OC activation has been fully established in PM osteoporosis and RA. Its final expression depends of the cytokine profile predominant in the circulating micromillieu. In an environment with a great inflammatory component, such as rheumatoid synovium, the expression of RANKL is increased through different stimulation pathways. It may also be induced through signals dependent on mechanical stimuli or hormonal deficits, such as PM or postimmobilization osteoporosis. In fact, the demonstration of the protagonic role of RANKL in these processes and their regulation by IFN-γ constituted the parting point of osteoimmunology.
The RANKL/RANK axis controls the development of lobar-alveolar cells in the mammary gland during gestation, through Ikk-α, a protein kinase necessary for the renovation of stem cells and also related to the progression of breast cancer and prostate cancer metastasis.71,72 The origin of RANKL under these circumstances had not been defined until Tan et al., in a mouse model for breast cancer, demonstrated that the main source of tumoral stroma were TREG CD4+ CD25+ FOXP3+ cells, traditionally associated to a poor prognosis, and that RANKL was implicated in the local extension and metastatic capacity of carcinomatous cells expressing RANK.73 The paracrine role of RANKL in breast cancer genesis has been confirmed in other studies with different methodology.74,75
In other circumstances, the relationship of RANKL with TREG cells also seems evident. For example, a mouse model of diabetes76 observed that the administration of these cells prevented the destruction of beta cells on the pancreatic isles, while inhibition of the RANKL/RANK axis inhibited the local accumulation of TREG cells, producing activation of cytotoxic T cells and destruction of beta cells. Something similar was seen in colitis, where RANKL inhibition stopped the expansion of regulating cells, worsening intestinal inflammation.77
The global study of the murine mutant phenotypes has also been a relevant source of information in the extraskeletal role of the RANKL/RANK/OPG axis. RANKL KO mice, in addition to presenting osteopetrosis and the absence of teeth, show a normal CD4+/CD8+ ratio, but both T cell activation as well as B cell development is altered. Defects in peripheral lymph nodes and a reduced size of Peyer's patches can also be seen, while the spleen structure is normal. There are no obvious OC and therefore, no erosions in this model of induced arthritis. When the RANK gene is silenced, results are similar. As for the OPG KO mice, the consequences are not symmetrically opposite. There is not only osteoporosis, with marked bone fragility, but are also arterial calcification and hearing loss, with data currently insufficient on the characteristics of the immune effectors in this model.78,79
Finally, RANKL expressed on T cells is necessary for the formation of medullary epithelial cells of the thymus, responsible for negative selection of autoreactive cells, a role whose relevance during the embryonic period is unquestionable, although its importance in the adult immune system is unknown.80
RANKL, in conclusion, is a cytokine that participates in the development of the immune system and breast tissue. Its role on the local extension and at-a-distance dissemination of certain cancers is very noteworthy and seems to be related to the negative function attributed to the infiltration of tumor stroma by TREG cells. In other organs and functions, its role is more obscure and may be tissue-specific, acting as a proinflammatory or anti-inflammatory molecule, in direct relationship to regulating cells.
Bone Cells as ImmunomodulatorsOne of the functions of the skeleton, enumerated in Table 1, is the support of vital structures, among them the bone marrow. Although classically considered as a simple protective structure, it is currently well demonstrated that bone tissue participates markedly on the marrow physiopathology, forming a part of the hematopoietic stem cells, intervening in decisions such as their fate, strongly dependent on the local micromillieu.
In 1994 it was first shown that OB maintained the proliferation of primitive hematopoietic progenitors in vitro, through G-CSF,81 and, since then, interest on the role of these cells in the fate of hematopoietic progenitors has grown, especially in the case of immune related cell lineages.82 A subclass of OB, called spindle-shaped N-cadherin+ osteoblastic cells due to their morphologic and genetic characteristics, have the functional capacity to interact with hematopoietic stem cells and structurally support their anchoring to the endosteal niches’ interior.83
Other signaling pathways, such as Notch84 and some molecules secreted by the OB, such as angiopoietin-1,85 have also been related to the regulation of the hematopoietic stem cell pool and the equilibrium of quiescent or development phase cells, albeit with contradictory results. It can be concluded that OB have a relevant role to play in the endosteal niche, although their precise function is not yet well defined.
OC, with their exclusive resorption function, could be the ones in charge of degrading the endosteal surface and building structurally adequate sites in which hematopoietic stem cells situate themselves. In this way, BR and hematopoiesis would be intimately connected. However, recent studies have been unable to demonstrate the necessary participation of OC in the maintenance and mobilization of stem cells, and their function remains purely structural.86 Other functions, such as the possible role they play in maintaining plasmatic cells, after the in vitro demonstration of an increase in the survival of these cells in the presence of OC, something that requires cell contact and BAFF and APRIL independent mechanisms,87 as well as antigen presenting functions88 with a capacity to activate T CD4+ and CD8+ cells, indicate the need for further studies that determine the exact role of OC in the regulation of the immune system.
Future PerspectivesIt is evident that grouping the knowledge that has arisen from different scientific disciplines and have related the immune system with the skeleton have been beneficial for the development of new therapeutic targets. For example, the enormous secretory capacity of the OS indicates that its functions transcend the bone and go much further that its role in BR. It is intriguing to observe the consequences of sclerotin gene suppression, taking into account the fact that it is a therapeutic target in a very advanced stage of clinical research. In a recent study performed in the SOST (−/−) mouse model, Cain et al. observed that the development of the B cell lineage was affected in all its phases due to an increase in these cells’ apoptosis.89 The analysis of the expression of this gene (whose product is sclerotin) confirmed its ostecyte origin and its absence in the hematopoietic lineage. In addition, the described alteration was independent of Wnt. We could say that the OS, through sclerotin, has a previously unknown role in the maturation of the B cell lineage maturation, whose study is of vital interest.
Another relevant aspect that extends even more the perimeter of osteoimmunology has been recently manifested by Buchwald et al.90 This group has previously shown that mice OC may recruit naïve CD8 cells under non-inflammatory conditions and activate them to induce T CD8 FoxP3+ (TcREG) lymphocytes and also produce cytokines such as IL-2, IL-6, IL-10 or IFN-γ,91 with individual actions over osteoclastogenesis which may be positive or negative. Through an in vitro study they determined that the net effect of these cells is suppressive and direct, inhibiting the differentiation of mature OC, which loose their resorptive capacity, without affecting their survival. While the induction of TcREG lymphocytes by OC is antigen-dependent, the suppression of OC by TcREG does not require antigen or restimulation. The blockade of the abovementioned cytokines would partially reduce their inhibitor effect. The confirmation of these developments would indicate a regulating osteoimmune feed-back in OC activation and that this subtype of regulating T cells would participate not only in the immune system and arthritis pathogenesis but also in skeletal homeostasis. These results show that there is a complex equilibrium between the direct actions of TcREG lymphocytes and the indirect ones, mediated by cytokines with antagonic functions at the individual level.92
Intercellular communication is another aspect that is becoming a first level therapeutic target.93 A new group of very interesting molecules is being added to the already known roles of the family of Ephrins, ligands bound to membrane and its Ephs receptors in signal transmission between OC and OB. These are the semaphorins, initially identified as an axonal guidance system necessary for neurons to reach their targets and later implicated in numerous organic functions, among them the immune system and the skeleton.94 There is no current molecule known to have a protective effect on the skeleton by acting locally on the OC and OB, impeding the breakup of the coupling between resorption and formation and leading different drugs to reach repeatedly a therapeutic ceiling due to the cessation of in osteoblastic activity that follows the use of antiresorptives and, on the other hand, to the osteoclastic stimulation that follows the use of anabolic treatments. Semaphorin 3 A (Sema 3 A) binds neurophillin 1 (NRP-1) blocking RANKL induced osteoclastic differentiation by inhibiting the proximal ITAM and RhoA signaling pathways, while simultaneously stimulating OB through the canonical Wnt pathway. Sema 3A −/− mice have an osteopenic phenotype that may be reproduced altering Sema 3A/NRP-1 signaling, while the intravenous infusion of this semaphoring increases bone volume and accelerates osteoregeneration.95
ConclusionsOsteoimmunology is a new scientific discipline which is rapidly evolving, studying the mutual effects of the skeleton and immune systems at the cellular and molecular levels, both in physiologic as in pathologic conditions. Osteoimmunology has shown that there exists an ample repertoire of molecular and cellular interactions whose detailed knowledge is providing a solid scientific basis for a paradigm shift in the field of osteoimmune diseases, allowing the development of more effective and safer therapeutic strategies in the coming years.
Ethical ResponsibilitiesProtection of People and AnimalsThe authors declare that this research has not been done in humans or animals.
Confidentiality of DataThe authors state that no patient data appear in this article.
Right to Privacy and Informed ConsentThe authors state that no patient data appear in this article.
Conflict of InterestThe authors declare no conflict of interest.
Term Glossary
Apc (adenomatous polyposis coli): Intracellular protein that, along with axin and GSK3 β, forming part of the complex retaining β-catenin and promoting its degradation. When the canonical Wnt pathway is activated, it is attracted to the cell membrane with the rest of the retaining structure, releasing β-catenin.
Atp6v0d2 and Atp6i: Isoforms of vacuolar ATPase that carries out important roles for the function of the proton pump that allows for the acidification of the osteoclastic lagoon. Its dysfunction can lead to severe forms of osteopetrosis.
ATF4 (activating transcription factor 4): Transcription form that carries out a relevant role in more mature osteoblastic lineage cells.
BAFF (B cell activating factor belonging to the TNF family): Cytokine implicated in the maturation and survival of peripheral B-lymphocytes and in the activation of B and T lymphocytes.
BAPX1 (bagpipe homeobox protein homologue 1): Transcription factor that stimulates osteoblastogenesis in the axial skeleton.
Bcl-2 (B-cell CLL/lymphoma 2): Antiapoptotic molecule that acts by inhibiting the liberation of mitochondrial cytochrome C, promoting the differentiation, activation and survival of osteoblasts and osteoclasts.
β-Catenin: Cytoplasmic multifunctional protein that mediates Wnt signaling.
BMP (bone morphogenic protein): Group of cytokines originally described in relation to their role as potent osteoforming inductors. Their current role is very ample, related fundamentally with morphogenetic signals in many tissues.
c-Fos: Transcription factor implicated in osteoblast differentiation. It dimerizes with c-Jun protein to form the AP-1 transcription factor, activating the transcription of numerous and diverse genes related to cell proliferation and differentiation.
Cadherins: Transmembrane glucoprotein family that mediates intercellular adhesion and act as signal receptors that affect cell differentiation and proliferation.
Catepsin K: Cistein-protease secreted by the osteoclast to the brush border and produces the first phase of bone matrix collagen dissolution.
CK1 (casein kinase 1): Kinase family that regulates signal transduction of multiple pathways, Wnt-β-catenin among others.
ClC7 (chloride channel 7): One of the most relevant chloride channel superfamily members, participating in chloride exchange, a process fundamental to osteoclastic resorption.
CREB (cAMP response element-binding): Transcription factor implicated in osteoclast activation.
DAP12 (DNAX-activating protein de 12kDa): Adaptor protein containing ITAM (immunoreceptor tyrosine-based activation motifs) that has an essential role in RANK signal transduction.
DC-STAMP (dendritic cell specific transmembrane protein): Transmembrane protein fundamental for osteoclastic fusion.
Dmp1 (Dentin matrix acidic phosphoprotein 1): Extracellular matrix protein with a very relevant role in the mineralization of bone and dentin.
Dsh (disheveled): Cytoplasmic phosphoprotein that plays a relevant role in the intracellular signaling of frizzled receptors.
Dkk (Dickkopf): Protein family composed by 4 members, containing between 206 and 366 aa. Dkk-1 and 4 inhibit the Wnt signaling pathways by binding LRP 6 through its CRD1 domain, making them natural antagonists of the canonical Wnt pathway.
FcR-γ (gamma Fc receptors): Receptors recognizing the Fc portion of IgG, important in leukocyte response to immune complexes, participating also in the osteoclast lineage.
FGF (fibroblast growth factor): Cytokine family that plays a key role in different cell proliferation and differentiation processes. 22 members have been identified in humans and its action is carried out through Ig-like extracellular domains and signal transduction through tyrosine-kinases.
Frizzled: A receptor of the 7 transmembrane domain family that transmits the intracellular signal of Wnt ligands. 10 members are recognized, numbered from 1 to 10, with a variable size oscillating between 500 and 700 aa. The cysteine rich domain of the extracellular aminoterminal region is highly conserved between species and is necessary for Wnt binding.
Gab-2 (GRB2-associated binding protein 2): Adaptor protein participating in the intracellular transmission of different signals in response to stimuli from cytokine and growth hormone receptors.
GH/IGF-1(growth hormone/insulin-like growth factor 1): Endocrine axis that plays a relevant role in osteoformation. There is a circulating form of IGF-1, secreted by the liver in response to GH and a local form that acts in multiple cell processes as an autocrine or paracrine factor.
GSK3 β (glycogen synthase kinase 3 beta): see Apc.
H+-ATPase: Osteoclast ATPase that allows the passage of protons through the membrane to acidify the lagoon and dissolve the calcified material.
IFN-γ (gamma interferon): cytokine produced mainly by T lymphocytes and NK cells whose most important function is macrophage activation, both in innate and acquired immune responses. It inactivates TRAF6 in the osteoclast, impeding RANK signal transduction and, in this manner, plays an antiosteoclastic role.
Integrins: Molecules expressed on the osteoclast membrane that facilitate their adhesion to mineralized tissue through the interaction with bone matrix proteins. Integrin a2β1 binds collagen, while integrin avβ3 does so with vitronectin, osteopontin and bone syaloprotein.
ITAM (intracellular tyrosine-based activation motif): Motifs present on different adaptors, among them TRAF6, important for RANK signal transduction.
Jnk (c-Jun N-terminal kinase): Belongs to the mitogen activated kinase family and has a relevant role in stress stimuli signals, among them inflammation.
Krm (kremen) Transmembrane proteins implicated in Wnt/Dkk signaling.
Lef1 (Lymphoid enhancer binding factor): Transcription factor involved in Wnt signaling.
Lrp 5/6 (low-density lipoprotein receptor-related protein 5/6): Transmembrane proteins belonging to the LDLR family, 1.615 and 1.613 aa in size respectively. In contrast to other members of the family, Lrp 5 and 6 act as frizzled coreceptors in the transmission of Wnt signals.
M-CSF (macrophage-colony stimulating factor): implicated in the initial stages of osteoclastic differentiation.
MITF (microphthalmia-associated transcription factor): Transcription factor participating, among other cell processes, in early stages of osteoclast differentiation.
NFATc1 (nuclear factor of activated T cells, cytoplasmatic): Transcription factor that acts as a master regulator of osteoclast differentiation and activation.
NIK (NF-κB inducing kinase): Kinase that participates in the activation of NF-κB.
OPG (osteoprotegerin): Also known as the 11B member of the TNF receptor superfamily, a cytokine that acts as a decoy for RANKL. After binding to it, it inhibits NF-κB and the activation of genes related with the immune response and osteoclast activation.
OSCAR (osteoclast-associated receptor): IgG-like receptor with no currently known ligand, mainly expressed on osteoclasts, monocytes, granulocytes, macrophages and dendritic cells. It participates in osteoclast activating signals in a manner that complements RANKL/RANK.
Osx (osterix): Main transcription factor that stimulates osteoblastic differentiation, acting distal to Runx2.
PCP (planar cell polarity): Alternative to the Wnt signaling pathway.
PU.1: Transcription factor regulating B cell function.
RANK (receptor activator of nuclear factor κB): Type i membrane receptor expressed on the surface of osteoclasts. Its ligand is RANKL.
Decoy receptors: Receptors that recognize cytokines ith an elevated affinity and specificity, but are structurally incapable of inducing signaling or presenting an agonist to the signal receptor complex. OPG (osteoprotegerin) is a decoy receptor that regulates remodeling by trapping RANKL and “protecting” the bone.
ROR 1 and 2 (tyrosine kinase orphan receptor): Belongs to the tyrosine kinase family (Trk) of receptors.
Runx2 (runt-related transcription factor 2): Master regulator and, also, the earliest one to appear in the process of osteoblastic differentiation. Necessary but not enough for a progenitor cell to differentiate.
SATB2 (special AT-rich sequence-binding 2): Extracellular matrix protein that stimulates osteoblastogenesis through Runx2.
Scl (sclerostin): Hormone secreted by mature osteocytes that plays an important role in bone remodeling, both for its antagonic action on the Wnt pathway as well as for its recently described autocrine and paracrine action on RANKL.
sFRP (secreted frizzled-related protein): Family composed by 5 members, of 286 and 325 aa, which are antagonists of the Wnt pathway, binding directly to the ligand, blocking interaction with frizzled.
SQSTM1/p62 (sequestosome 1): Signal modulator implicated in receptor mediated signal transduction. In the osteoclast it participates in the autophagic degradation of the NF-κB inhibitor, allowing cell activation.
SOST (sclerostin gene): gene encoding sclerostin.
TCF/Lef (cell-specific transcription factor/lymphoid enhancer binding factor): Transcription factor family involved in Wnt signaling.
TGF-β (transforming growth factor 1): Multifunctional protein with a relevant role in the proliferation and differentiation of diverse cell types.
TRAP (tartrate-resistant acid phosphatase): Enzymatic marker of mature osteoclasts.
TRAF6 (TNF receptor associated factor 6): Adaptor protein with a critical transduction function in the intracellular signaling of members of the IL-1R/TLR and TNFR superfamilies.
TREM2 (triggering receptor expressed in myeloid cells-2): Receptor without a known ligand with an IgG-like structure that participates, as does OSCAR, in osteoclast costimulation.
Basic multicellular unit (BMU): Group of cells coordinately participating in remodelling.
Wif (Wnt inhibitory factor): Natural Wnt ligand antagonist.
Wise (Wnt modulator in surface ectoderm): Wnt signal modulator.
Wnt: Glycoprotein family secreted and post-transcriptionally modified through the addition of lipids (palmitate). They act as natural ligands, unleashing multiple signaling cascades that intervene in key processes of embryonic development and tissue regeneration. Very unstable and difficult to isolate. In mammals there are currently 19 different Wnt proteins described, acting on 4 different signaling pathways. The Wnt-β-catenin pathway is the best characterized and has a key role in osteoblast development and function.
Please cite this article as: Arboleya L, Castañeda S. Osteoinmunología: el estudio de la relación entre el sistema inmune y el tejido óseo. Reumatol Clin. 2013;9:303–315.