This work reports patient treatment survival and adverse events related to Biologic Therapy (BT), identified by a multicenter ambispective registry of 2047 rheumatic patients undergoing BT and including a control group of Rheumatoid Arthritis (RA) patients not using BT. The most common diagnoses were: RA 79.09%, Ankylosing Spondilytis 7.96%, Psoriatic Arthritis 4.40%, Systemic Lupus Erythematosus 3.37%, Juvenile Idiopathic Arthritis 1.17%. A secondary analysis included 1514 cases from the total sample and was performed calculating an incidence rate of any adverse events of 178×1000/BT patients per year vs 1009×1000/control group patients per year with a 1.6 RR (95% CI 1.4–1.9). For serious adverse events the RR was: 15.4 (95% CI 3.7–63.0, P<.0001). Global BT survival was 80% at 12 months, 61% at 24 months, 52% at 36 months and 45% at 48 months and SMR: 0.23 (95% CI 0.0–49.0) for BT vs 0.00 (95% CI 0.0–0.2) for the control group. In conclusion, BT was associated to a higher infection risk and adverse events, compared to other patients. Mortality using BT was not higher than expected for general population with same gender and age.
Mediante registro multicéntrico ambispectivo de 2.047 pacientes con diversas afecciones reumáticas bajo terapia biológica (TxB), incluyendo un grupo control de pacientes con artritis reumatoide (AR) sin TxB, se reporta la supervivencia en la terapia y eventos adversos asociados a su uso. Los diagnósticos más frecuentes son: AR 79,09%; espondilitis anquilosante (EA) 7,96%; artritis psoriásica (APso) 4,40%; lupus eritematoso sistémico (LES) 3,37% y artritis idiopática juvenil (AIJ) 1,17 por ciento. Un análisis de 1.514 casos de la muestra total reportó que la tasa de incidencia para cualquier evento adverso es de 178/1.000 pacientes-año en TxB vs. 109/1.000 pacientes-año en controles con un riesgo relativo (RR) de 1,6 (IC del 95%, 1,4-1,9); para eventos adversos graves un RR de 15,4 (IC del 95%, 3,7-63,0 p<0,0001). La supervivencia global de TxB es del 80% a 12 meses, el 61% a 24 meses, el 52% a 36 meses y el 45% a 48 meses. La tasa de mortalidad estandarizada (TME) es de 0,23 (IC del 95%, 0,0-49,0) para TxB vs. 0,00 (IC del 95%, 0,0-0,2) para controles. Se concluye que la TxB se asocia a un mayor riesgo de presentar eventos adversos, especialmente infecciosos, en comparación con pacientes sin TxB. La mortalidad de los pacientes expuestos a TxB no es mayor que la esperada para la población general ajustada a edad y sexo.
Rheumatic diseases affect more than 10% of the general population.1 Mexico has identified a high prevalence of major rheumatic diseases: in particular, the prevalence of rheumatoid arthritis is estimated 1.60% (95% CI 1.4–1.7),2 in our environment, and is one of the main demands of attention in primary care and a cause of the high cost of health3 services.
The treatment of these diseases is complex, since it requires multidisciplinary and early management which should include nonpharmacologic measures, such as physical, occupational and psychological and drug treatments with different mechanisms of action, such as the chronic use of analgesics, nonsteroidal antiinflammatory drugs (NSAIDs) and even glucocorticoids. The cornerstone of management is the early introduction of disease modifying drugs (DMARDs) such as methotrexate, antimalarials, sulfasalazine and leflunomide.4,5 During the last decade the options of DMARDs have increased, with the development of promising new drugs, including biological agents that usually consist of monoclonal antibodies directed to specific molecules such as tumor necrosis factor (anti-TNF-α), interleukin receptor (IL-1 and IL-6), B cells and regulators of T cell costimulation6 and many more in development.
Numerous clinical trials have demonstrated the efficacy and safety of biologic7 therapy, but long and medium term follow-up of these cases has been limited.8 Consequently, assessments in real scenarios are necessary to determine the efficacy and safety of this drug class in clinical practice and to this end different registries have been carried out,9,10 mostly in developed countries, but the results have been highly variable, due to different methodologies and analysis, plus they should consider the demographic, socioeconomic and medical care of each society, especially those with emerging economies, where there are important differences to biological therapy, such as cost and access of these drugs. In addition, the epidemiological scenario is different from that reported in developed societies regarding an increased risk of infections. Therefore, the purpose of this report is to describe the adverse events reported in a cohort of Mexican patients with rheumatic diseases treated with biologic agents and identify the survival rate of the same and causes of discontinuation of therapy.
MethodsThe platform for the registration of patients using biological therapy is that of the Spanish Society of Rheumatology (SER), used since 2000,11 considered by PANLAR Biobadamérica for development of the project, whose main objective is to get local records relating to the use of biologics in Latin America and in which each country adopts the registry with appropriate modifications.
The Mexican College of Rheumatology (CMR) initiated a retrospective registry (RECOLBI) in 2007 of cases of patients with rheumatic diseases treated by specialists in rheumatology with biologic drugs, and since 2008 has used the BIOBADASER Biobadamex12,13 platform, which also included a control group of patients with the diagnosis of rheumatoid arthritis (RA) treated with non-biological DMARDs. This was possible due to the different health care systems in the country, because not all systems covered biological therapy, such as some hospitals of the Ministry of Health as well as private care, where access depends on the direct purchase of treatment by the patient.
Patients with any kind of rheumatic disease that warranted the use of biologic therapy were considered candidates to enter the registry. Minimum follow up time in order to be added to the registry was one year from the date of onset of biological drug. The protocol was approved and registered (2009-785-103) in the Mexican Social Security Institute.
Certified rheumatologists in the country were invited to participate in the registry and include a minimum of 20 cases with no maximum. After remote (by telephone or via the Internet) or face to face training, each researcher was assigned a password; in this way, each specialist registered cases diagnosed and treated with biological agents online at their leisure (except for cases that were included in treatment protocols or were part of a clinical trials) who had at least one year of follow-up. For quality control we carried out an online review of 100% of the cases and then subjected them to monitoring. Each center was monitored locally, and a monitor visited the center to randomly check a sample of at least 25% of cases. If more than 25% of inconsistencies were found, the case was removed from the registry.
Baseline: the registry consists of an electronic platform in which baseline data such as demographic characteristics were entered: age, sex, education, healthcare system, clinical parameters of the disease: diagnosis, duration, activity in cases of RA (DAS28) and ankylosing spondylitis (BASDAI), comorbidity, prior treatment for rheumatic disease and concomitant therapy, as well as information related to the biological agent: type of drug (adalimumab, abatacept, etanercept, infliximab, rituximab), drugs available in Mexico during this phase of registration, also including the date of onset and discontinuation of treatment and the cause in case of discontinuation. Each patient attended a scheduled appointment with the attending rheumatologist and was followed with a frequency individualized according to the discretion of each physician, without following previously set intervals.
In this study, all adverse events considered harmful or undesirable after the administration of a drug, at doses commonly used to prevent, diagnose or treat a disease, or to modify any biological function were registered. Serious adverse event was defined as any unfavorable event, regardless of dose, with life-threatening characteristics, which required hospital admission or prolonged hospitalization, as well as producing persistent or significant disability; a fatal adverse event was every unfavorable event regardless of dose, that was fatal. We used the nomenclature of MedDRA (Medical Dictionary for Drug Regulatory Activities) to classify each of the adverse events.
During follow-up, evaluations of adverse events associated with the use of biologic therapy were recorded and, if they occurred, changes in biological treatment schemes were filed. Minimum follow-up of the patient in order to report adverse events or changes in the biologic was of one year. Because the temporary registration was both retrospective and prospective, if a patient already had a one-year period on the biological drug, the patient could be considered in the analysis, but if the time to include the patient still did not meet this interval, patient follow-up continued for at least 12 months.
Statistical analysis: This report includes a first descriptive analysis of 2047 patients at baseline through February 2011, considering their demographic information, clinical features and treatment. The second phase of analysis consisted of 1514 of the total 2047 cases, which correspond to cases monitored, including in this group those receiving biological therapy (n=1114) vs a control group of patients with RA treated with non-biological DMARDs (n=400), analyzing the results using the Student t test, ANOVA for continuous variables and χ2 test for qualitative variables. This same subsample of 1514 cases was used to identify the risk of an adverse event between the biological vs. non-biological group treated with DMARDs alone; we calculated the incidence rate of adverse events per 100000 patient-years (95%). Standardized rates were calculated adjusted for age and sex in general Mexican population. Finally, survival with the drug was identified through the Kaplan–Meier method, including those treated with anti-TNF, considering variable census date of discontinuation of the biological (independent of the cause). The analysis was performed using the STATA statistical software. Statistical significance was assumed with P values<.05.
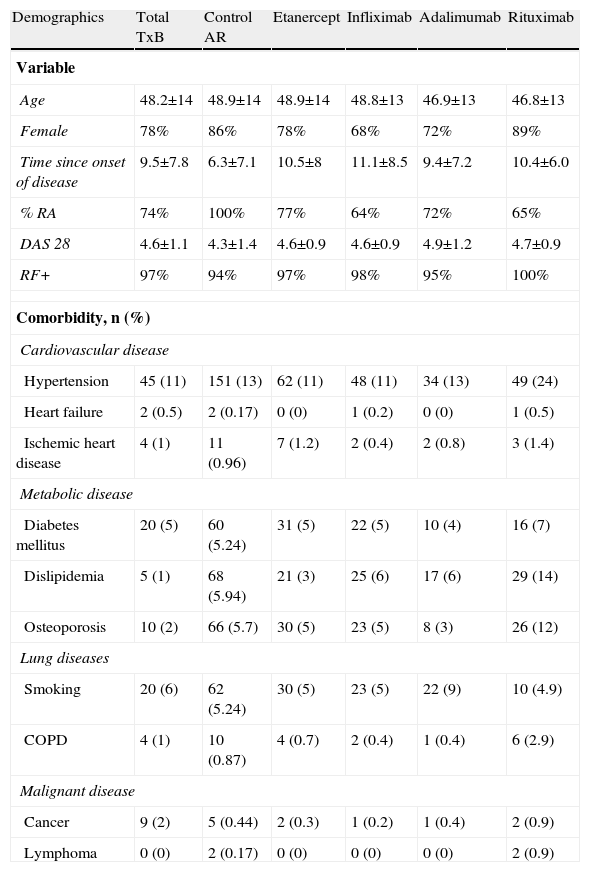
ResultsBiobadamex 1.0 (February 2009–February 2011) involved 25 centers and 46 rheumatologists; 2047 patients were registered, with an average age of 49.5±14 years, 73% of cases corresponded to females; the mean disease duration of 10±8 years. Most patients were treated in one of the social security system in Mexico (IMSS, ISSSTE, PEMEX, ISSEMYM and Ministry of Health). Only 9.05% of them were in private care. Among the total 2047 patients, a total of 2651 treatments were performed, distributed as follows: 1916 correspond to biological therapy and 735 (27.73%) were cases of RA treated with nonbiological DMARDs, considered as control group. The general demographic characteristics and comorbidities of the total population and by treatment received are seen in Table 1.
General Characteristics of the Biobadamex 1.0 Registry.
| Demographics | Total TxB | Control AR | Etanercept | Infliximab | Adalimumab | Rituximab |
| Variable | ||||||
| Age | 48.2±14 | 48.9±14 | 48.9±14 | 48.8±13 | 46.9±13 | 46.8±13 |
| Female | 78% | 86% | 78% | 68% | 72% | 89% |
| Time since onset of disease | 9.5±7.8 | 6.3±7.1 | 10.5±8 | 11.1±8.5 | 9.4±7.2 | 10.4±6.0 |
| % RA | 74% | 100% | 77% | 64% | 72% | 65% |
| DAS 28 | 4.6±1.1 | 4.3±1.4 | 4.6±0.9 | 4.6±0.9 | 4.9±1.2 | 4.7±0.9 |
| RF+ | 97% | 94% | 97% | 98% | 95% | 100% |
| Comorbidity, n (%) | ||||||
| Cardiovascular disease | ||||||
| Hypertension | 45 (11) | 151 (13) | 62 (11) | 48 (11) | 34 (13) | 49 (24) |
| Heart failure | 2 (0.5) | 2 (0.17) | 0 (0) | 1 (0.2) | 0 (0) | 1 (0.5) |
| Ischemic heart disease | 4 (1) | 11 (0.96) | 7 (1.2) | 2 (0.4) | 2 (0.8) | 3 (1.4) |
| Metabolic disease | ||||||
| Diabetes mellitus | 20 (5) | 60 (5.24) | 31 (5) | 22 (5) | 10 (4) | 16 (7) |
| Dislipidemia | 5 (1) | 68 (5.94) | 21 (3) | 25 (6) | 17 (6) | 29 (14) |
| Osteoporosis | 10 (2) | 66 (5.7) | 30 (5) | 23 (5) | 8 (3) | 26 (12) |
| Lung diseases | ||||||
| Smoking | 20 (6) | 62 (5.24) | 30 (5) | 23 (5) | 22 (9) | 10 (4.9) |
| COPD | 4 (1) | 10 (0.87) | 4 (0.7) | 2 (0.4) | 1 (0.4) | 6 (2.9) |
| Malignant disease | ||||||
| Cancer | 9 (2) | 5 (0.44) | 2 (0.3) | 1 (0.2) | 1 (0.4) | 2 (0.9) |
| Lymphoma | 0 (0) | 2 (0.17) | 0 (0) | 0 (0) | 0 (0) | 2 (0.9) |
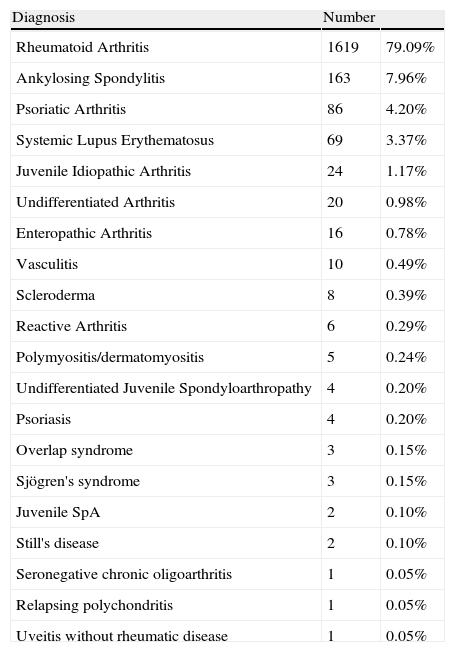
The most frequent diagnosis was RA (79%) followed by seronegative spondyloarthropathies (14.4%), including in these the following entities: ankylosing spondylitis, psoriatic arthritis, undifferentiated spondyloarthropathy, enteropathic arthritis, juvenile onset spondyloarthropathy and seronegative chronic oligoarthritis; the other diagnoses treated with biologic therapy are listed in Table 2.
Diagnosis of the Patients in the Biobadamex 1.0 Registry.
| Diagnosis | Number | |
| Rheumatoid Arthritis | 1619 | 79.09% |
| Ankylosing Spondylitis | 163 | 7.96% |
| Psoriatic Arthritis | 86 | 4.20% |
| Systemic Lupus Erythematosus | 69 | 3.37% |
| Juvenile Idiopathic Arthritis | 24 | 1.17% |
| Undifferentiated Arthritis | 20 | 0.98% |
| Enteropathic Arthritis | 16 | 0.78% |
| Vasculitis | 10 | 0.49% |
| Scleroderma | 8 | 0.39% |
| Reactive Arthritis | 6 | 0.29% |
| Polymyositis/dermatomyositis | 5 | 0.24% |
| Undifferentiated Juvenile Spondyloarthropathy | 4 | 0.20% |
| Psoriasis | 4 | 0.20% |
| Overlap syndrome | 3 | 0.15% |
| Sjögren's syndrome | 3 | 0.15% |
| Juvenile SpA | 2 | 0.10% |
| Still's disease | 2 | 0.10% |
| Seronegative chronic oligoarthritis | 1 | 0.05% |
| Relapsing polychondritis | 1 | 0.05% |
| Uveitis without rheumatic disease | 1 | 0.05% |
Treatment recorded at the time of initiating biological therapy were: 77.29% and 42.29% methotrexate and corticosteroids, followed by antimalarials, 26.59%, leflunomide, 21.65%, sulphasalazine, 21%, 65%, and less than 5% of cases azathioprine, cyclophosphamide, cyclosporin and mesalazine. 40.36% of the patients reported the presence of comorbidity, the most frequent being: hypertension, 14.95% (n=306), diabetes, 5.81% (n=119), osteoporosis, 5, 32% (n=109), and smoking, 5.18% (106). In addition to DMARDs, the concomitant therapy most frequently reported were: NSAID 39.62% (n=437), folic acid 31.82% (n=351), paracetamol 7.89% (n=87), omeprazole 6.89% (n=76), other medications such as calcium, vitamin D, bisphosphonates, oral antidiabetics, and antidepressants were reported in less than 3% of cases.
The most widely used biological drugs for the management of rheumatic disease in Mexican registration are anti-TNF-α (60%), with the following distribution: etanercept 25.61% (679), infliximab 19.80% (525) and adalimumab 14.56% (386). Other biological agents such as rituximab were used in 10.60% (281) and 1.20% employed abatacept (34).
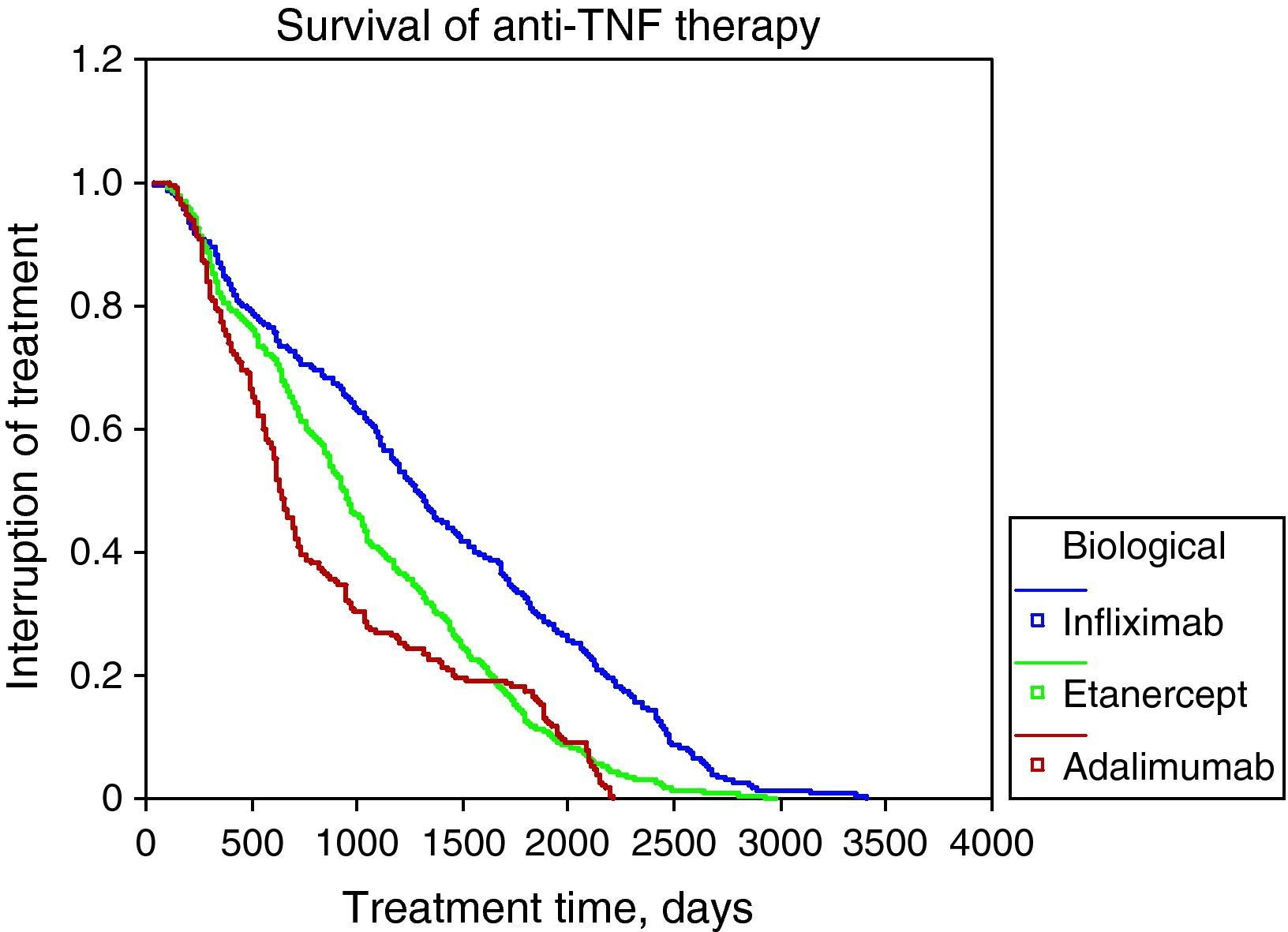
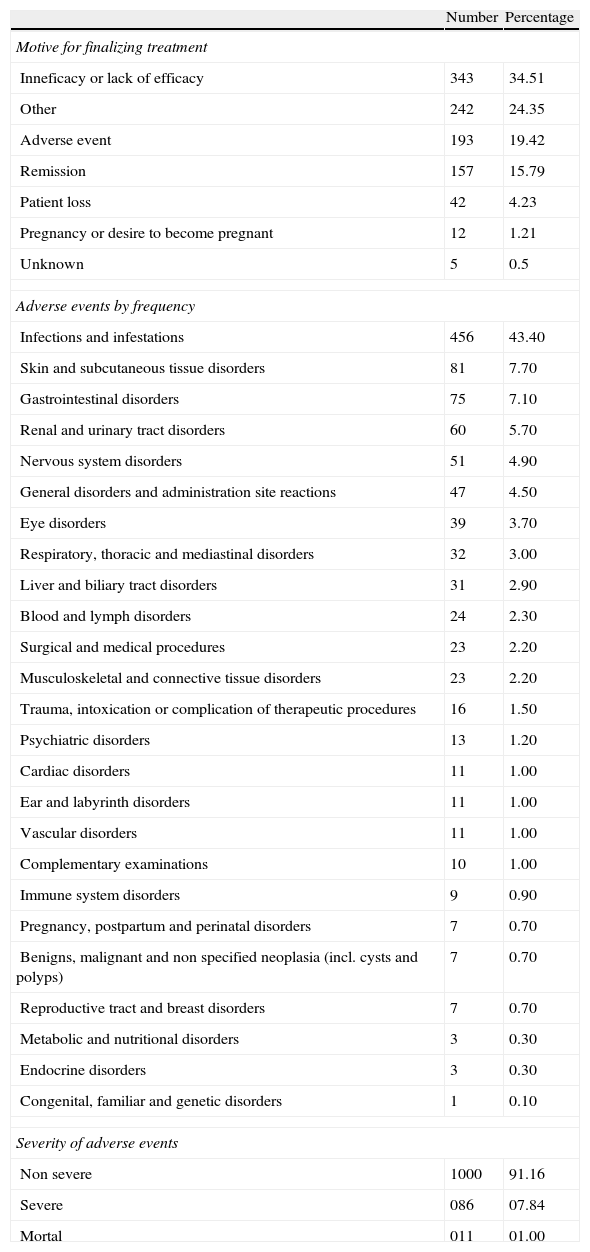
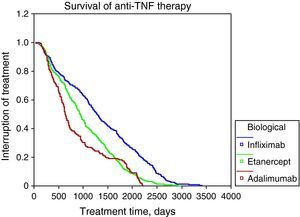
The survival rate of biological therapy globally to 12 months of initiation was 80%, 24 months to 36 months 61%, 48 months 52% and 45%. The most frequent causes of discontinuation of anti-TNF-α were: lack of efficacy (56%), adverse events (5%). Figure 1 shows the survival curves of the 3 anti-TNF-α in Table 3 and Table 3 shows the causes of discontinuation of all biological drugs, with 34.51% due to inefficiency or loss of the efficiency (n=343) and 24.35% due to adverse events (n=242) the most frequent.
Causes of Discontinuation of Biologic Therapy.
| Number | Percentage | |
| Motive for finalizing treatment | ||
| Inneficacy or lack of efficacy | 343 | 34.51 |
| Other | 242 | 24.35 |
| Adverse event | 193 | 19.42 |
| Remission | 157 | 15.79 |
| Patient loss | 42 | 4.23 |
| Pregnancy or desire to become pregnant | 12 | 1.21 |
| Unknown | 5 | 0.5 |
| Adverse events by frequency | ||
| Infections and infestations | 456 | 43.40 |
| Skin and subcutaneous tissue disorders | 81 | 7.70 |
| Gastrointestinal disorders | 75 | 7.10 |
| Renal and urinary tract disorders | 60 | 5.70 |
| Nervous system disorders | 51 | 4.90 |
| General disorders and administration site reactions | 47 | 4.50 |
| Eye disorders | 39 | 3.70 |
| Respiratory, thoracic and mediastinal disorders | 32 | 3.00 |
| Liver and biliary tract disorders | 31 | 2.90 |
| Blood and lymph disorders | 24 | 2.30 |
| Surgical and medical procedures | 23 | 2.20 |
| Musculoskeletal and connective tissue disorders | 23 | 2.20 |
| Trauma, intoxication or complication of therapeutic procedures | 16 | 1.50 |
| Psychiatric disorders | 13 | 1.20 |
| Cardiac disorders | 11 | 1.00 |
| Ear and labyrinth disorders | 11 | 1.00 |
| Vascular disorders | 11 | 1.00 |
| Complementary examinations | 10 | 1.00 |
| Immune system disorders | 9 | 0.90 |
| Pregnancy, postpartum and perinatal disorders | 7 | 0.70 |
| Benigns, malignant and non specified neoplasia (incl. cysts and polyps) | 7 | 0.70 |
| Reproductive tract and breast disorders | 7 | 0.70 |
| Metabolic and nutritional disorders | 3 | 0.30 |
| Endocrine disorders | 3 | 0.30 |
| Congenital, familiar and genetic disorders | 1 | 0.10 |
| Severity of adverse events | ||
| Non severe | 1000 | 91.16 |
| Severe | 086 | 07.84 |
| Mortal | 011 | 01.00 |
The 11 deaths reported in the table correspond to those registered in the total number of patients (2047). In the 1514 case subgroup, 6 deaths were reported.
During follow-up of patients, there were a total of 1097 adverse events among the 2651 total treatments. According to the MedDRA terminology, 91.16% of them were not severe, 7.84% were severe and 1.00% resulted in death. The most common recorded among all treatments were: infections and infestations in 43.40%, gastrointestinal disorders in 7.10%, skin and subcutaneous tissue problems in 7.70% and nervous system disorders in 4.90%. The list with the frequency of adverse events by type of organ or systems, and the frequency of fatal adverse events is presented in Table 3.
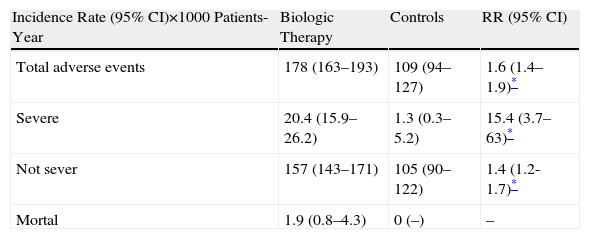
Of the 2047 total cases included in the second part of the analysis, 1514 cases (1114 biological therapies vs 400 controls) were used to calculate the incidence rate and relative risk of adverse events. The rate for any adverse event was 178/1000 patient years in the biological group vs 109/1000 patient years in the control group; for serious adverse events, it was 20/1000 patient-years vs 1.3/1000 patient-years; for fatal events it was 1.9/1000 patient-years vs 0/1000 patient-years. Exposure to any biological treatment was associated with an increased risk of adverse events of any kind (RR 1.6, 95% CI 1.4–1.9, P<.0001). The risk associated with use of biological drugs increased substantially when analyzing only serious adverse events (RR 15.4, 95% CI 3.7–63.0, P<.0001). Because no fatal adverse event in the control group were seen, it was not possible to calculate the RR (Table 4).
Relative Risk of Adverse Events According to Severity.
With respect to major adverse events according to MedDRA, there was an increased risk for infections and infestations (RR 2.05, 95% CI 1.5–2.7, P<.0001); in the 71.96% the germ was not identified and there were 8 cases of tuberculosis. The risks for other adverse events: general disorders and administration site reactions (RR 5.5, 95% CI 1.7–18.0, P<.001), respiratory, thoracic and mediastinal disorders (RR 4, 9, 95%, from 1.5 to 21.0, P<.05), CNS disorders (RR 3.3, 95% CI 1.4–7.9, P<.0001) and surgical procedures (RR 15.4, 95%, from 3.7 to 63.0, P<.0001), but not for other events. The only event with a lower RR in patients with biological compared to control group was for surgical procedures (RR 0.2, 95% CI 0.0–0.6, P<.01).
11 deaths were reported among the total registered patients (2047), but the standardized mortality ratio was calculated at 1514 cases, with 6 deaths reported, with full traceability of 3063 persons/year (all had received vs biological therapy. 0 in the control group) which, according to official data issued by the National Institute of Statistics, Geography and Informatics (INEGI) for total mortality for the general population, led to an estimated 26 cases of deaths, with a standardized mortality ratio of 0.23 (95% CI 0.0–49) vs 0.00 (95%, from 0.00 to 0.24) in the control group.
DiscussionNumerous publications have reported the long-term effects and safety of biologic therapy, including anti-TNF-α.8,11,14–16 Most of these reiterate the effectiveness and the potential to change the course of the disease, which promotes a better quality of life. On the other hand, reports record results that include thousands of cases, and while indicating a good overall safety profile, also demonstrate an increased risk of adverse events, mainly infectious.9,11,18 Most of these records have been conducted in developed countries and with Anglo-Saxon population, and only recently have the results of a Brazilian study been published (Biobadabrasil)19 and now we present our results in Mexico.
In this multicenter observational registry that analyzed data of 2047 Mexicans patients treated with biologics, we found a high percentage of treatment retention with anti-TNF-α (80% at one year and up to 45% at 48 months). By comparing retention rates with those reported at one and three years by the European groups such as Italy, 78.8% and 70%,16 Spain, 83% and 70%,17 and Sweden reported at 20 months that 79% persisted with anti-TNF-α and also observed a greater than 75% survival. The Brazilian registry reported 750 cases treated with anti-TNF and identifies which patients with RA are those that remain under treatment as well as an overall retention rate of 60% at 4 years.19 Also the causes of discontinuation are similar to those reported in this series, which in our population was 56% for lack of efficacy and 5% due to adverse events.
Regarding adverse events when analyzing a subset of the total sample vs the group of patients with RA treated without biological therapy, we found a rate of 178 adverse events vs 109/1000 patient-years among the group of biological vs controls and an increased risk of adverse events, the most common were infections, gastrointestinal disorders, skin, subcutaneous tissue, nervous system disorders and administration site reactions.
Lacaille20 reported the relative risk of infections in a cohort of patients with RA managed with non-biological DMARDs and followed for 162,710 person-years (RR 0.90, 95%, from 0.88 to 0.93), which increased with use of glucocorticoids to 0.92, 95%, from 0.85 to 1.0. In the Biobadamex registry, the estimated RR was 2.0 (95% CI 1.5–2.7, P<.0001), significantly higher and in agreement with other registries such as Biobadaser,21,22 which also has reported higher incidence rates of infections (114/2644 patient-years among patients with biologic therapies 63/2269 vs patient-years in the control group), but these incidents do not impact on outcomes such as mortality,23 although in our population there is an increased risk of infectious events in patients receiving biological therapy, it is not greater than that reported in series from developed countries despite the fact that in our country there is a greater epidemiological risk for infectious processes, supporting the safety profile for use of these drugs in our patients. In particular, episodes of tuberculosis are more common in our environment, and as the Spanish reports indicate, the control of potential risk factors is essential in limiting this adverse event.21 Aware of the high prevalence of tuberculosis in our environment, experts seem to be cautious and adhere to established recommendations before using an anti-TNF-α, even when facing a possible latent TB and even when associating preventive22 therapy.
Adebajo and Furst24 suggest making special considerations in the management with biological therapy in societies with emerging economies such as establishing registries, correctly interpreting adverse events, establishing appropriate outcome measures, taking into account the epidemiological setting in the risk of infection and the local cost as well as the different health systems that determine the access to biological therapy.
There are some limitations regarding the registry: the first is the need to expand, because although there is a sufficient number to perform a significance analysis, the inclusion of a larger number of cases in order to have representation from all regions of the country is required, and in this case, most patients are from the Mexican Social Security Institute and the center of the country (City and greater metropolitan area); the inclusion of patients not being managed by rheumatologists should also be considered. Thus, having a representative sample will identify with more precision the risk of infections such as tuberculosis that in this analysis does not appear to be higher than expected; another important factor is the difficulty in monitoring which is important in order to promote a culture of drug-surveillance in our routine practice to generate important information; others would be to include more specific outcome measures and the perspective of the patients,25 including aspects such as health and labor productivity measures, would assess the true effectiveness of this expensive therapy. These limitations may be partly due to the registry cohort design.
This is the first report in Mexico that allows us to know the characteristics of patients with rheumatic diseases treated with biologic therapy. Biobadamex is an example of some of the considerations proposed by Adebajo24 because it is a specific record of our country, the result of an interagency effort coordinated by the Mexican College of Rheumatology and whose aim is to obtain fundamental information at the national level (which was lacking) to be applicable to multiple levels: from health care to clinical decisions, to the institutional and social levels to consider management decisions and health policies, including valuable data for pharmaceutical companies and patients.
FinancingThe Mexican College of Rheumatology wishes to thank Bristol-Meyer Squibb, Pfizer, Roche, Schering-Plough (now Janssen-Cilag) and UCB, for the unrestricted financial support given to the project, developed with this support and the College's own resources.
Conflict of InterestThe authors of this study as well as the collaborators appearing in Annex 1 of coauthors have no disclosures to make.
| Salvador Salinas Saldivar | Guadalupe Aguilar Roca |
| Silvia Sánchez Alonso | Natasha Castro Lizano |
| Aida Galicia López | Angélica Peña Ayala |
| María del Carmen Sahagún Godínez | Ana Guilaisne Benard Medina |
| Jose Eduardo Navarro Zarza | Adolfo García González |
| Everardo Alvarez Hernández | Julio Cesar Casasola Vargas |
| Jesús Espinosa Villalpando | Juan Manuel Miranda Limón |
| Mario Pérez Cristóbal | Yeremi Díaz González |
| Gerardo Bori Segura | Brenda Roxana Vázquez |
| Ricardo Solórzano Acosta | Leonardo Limón Camacho |
| Claudia Irene Meléndez Mercado | Iliana Gabriela Holguín Dorador |
| Gustavo Esteban Lugo Zamudio | Virginia Alvarado Romano |
| Aleni Alieta Paz Viscarra | Ana Marcela Pizaña Serna |
| Carolina Duarte Salazar | María de los Angeles Díaz Ceballos |
| Leobardo Terán Estrada | Jorge Alberto Barragán Garfías |
| Ana Laura Marines Castillo | Mario Alfredo Chavez López |
| Francisco Antonio Rojo Leyva | Iván Francisco Chavira Ruiz |
| Rosa Elda Barbosa Cobos | Ana Sofía Vargas |
| Luis Javier Cruz Alvárez | Zully Castro Colín |
| Claudia Mendoza Pinto | Antonio Rodríguez Aguirre |
Please cite this article as: Ventura-Ríos L, et al. Terapia biológica: sobrevida y seguridad en padecimientos reumáticos. Resultados del Registro Nacional Biobadamex 1.0. Reumatol Clin. 2012;8:189–94.