Inflammatory myopathies are a heterogeneous group of myopathies in which there is biopsy-evident inflammation. In its evaluation it is essential to use neurophysiological techniques that provide information on the nature of the process.
This paper reviews the electromigram pattern characteristic of inflammatory myopathies, its diagnostic value, limitations, and some clues on the interpretation of the results of neurophysiological techniques in the assessment of inflammatory myopathies.
Las miopatías inflamatorias son un grupo heterogéneo de miopatías en las que existe inflamación en la biopsia. En su evaluación es esencial el uso de técnicas neurofisiológicas que permiten obtener información sobre la naturaleza del proceso.
Este trabajo revisa el patrón electromiográfico característico de las miopatías inflamatorias, su valor diagnóstico, las limitaciones y algunas pistas sobre la interpretación de los resultados de las técnicas neurofisiológicas en la evaluación de las miopatías inflamatorias.
Inflammatory myopathies (IM) are a heterogeneous group of autoimmune muscle diseases that include polymyositis, dermatomyositis and inclusion body myositis (MCI).1–4 They can occur alone or are associated with other autoimmune diseases and usually present elevation of muscle enzymes and predominantly proximal symmetric loss of strength.
Although IM seems to be a clinically defined entity with a predominantly degenerative pathogenesis, subsequent classifications traditionally included biopsy inflammation.5
The diagnosis of IM is based on clinical data, physical examination, the elevation of muscle proteins in the blood, determination of specific antibodies, neurophysiological tests, imaging tests and pathology of muscle obtained by biopsy.
Neurophysiological tests include studies of electroneurography or nerve conduction and needle electromyography (EMG) studies and they allow the exploration of the peripheral nervous system function, including nerves, neuromuscular junctions and muscles.
The utility of neurophysiological studies lies in its high sensitivity and specificity for the diagnosis of myopathies and the ability to exclude other causes of weakness such as neuropathy with motor involvement, myasthenic syndromes or motor neuron disease.
As a general rule, electromyographic study information usually begins to show about 2–3 weeks into the pathogenic process, so that in acute myopathies it is recommended to conduct the study about 3 weeks from the onset of symptoms to ensure good sensitivity.
The sensitivity of neurophysiological tests in IM has not been the subject of many studies. In the classic work of Bohan and Peter, the sensitivity of these was 89%.6 Therefore, it must be remembered that EMG may be normal, depending on the completeness of the study and the number of muscles explored, the timing and the ability of patient cooperation, the experience of electromyographic technician and so on.
EMG selects the muscle to be biopsied. Because needle electromyography injures muscle tissue, it is not advisable to biopsy muscles studied using this technique. We generally tend to perform the clinically weak muscle biopsy on the side opposite to where the EMG was performed and where it showed muscle electrical alterations. The correlation between electromyographic findings and a histopathological myopathy is high, 70%–90%.7
Performance of the EMG can increase blood levels discreetly, mainly creatine phosphokinase (CPK) and other muscle enzymes. It is therefore appropriate, if necessary to make a determination of CPK, to withdraw before completion of the EMG or at least 3 days after the EMG. If done immediately following the study, the possible elevation of muscle enzymes due to traumatic injury should be taken into account.8
Neither antiplatelet dugs nor anticoagulation are considered an absolute contraindication to perform an EMG.9,10 However, in over-anticoagulated patients with severe risk of clotting, the risk-benefit must be weighed and avoidance of hard or deep muscle compression in case of bleeding carried out.
Neurophysiological Protocol for the Study of Inflammatory MyopathiesIn general, at least one motor and sensory conduction of an affected limb should be studied, and in needle electromyography of affected muscles, at least one proximal and one distal muscle examined.11 It is also advisable to conduct a study of repetitive stimulation in cases with a suspected neuromuscular junction disorder.
Any muscle can be studied, but it is advisable to follow a systematic rationale. Clinically weak muscles and upper and lower extremities should be explored. If necessary, paraspinal and bulbar muscles can be explored. In general, the findings show electroneurographic myopathy with a normal nerve conduction, a possible decrease in the amplitude of motor potentials when the myopathy is either distal, severe, or evolving.
EMG is the most important neurophysiological study. It allows to study the electrical activity of muscle with concentric needle electrodes inserted into the muscle and detects extracellular potential differences. The study of the muscle is done in 2 situations: at rest and voluntary activity.
Resting values are called spontaneous activity. In normal muscle there should be no spontaneous activity, except for the noise called plaque. This is a sound similar to a seashell put next to the ear and represents the activity of depolarization of the endplate by the basal release of acetylcholine.
Abnormal or pathological spontaneous activity may be of several types:
- 1.
Fibrillations and positive waves.
- 2.
High frequency discharges.
- 3.
Myotonic discharges.
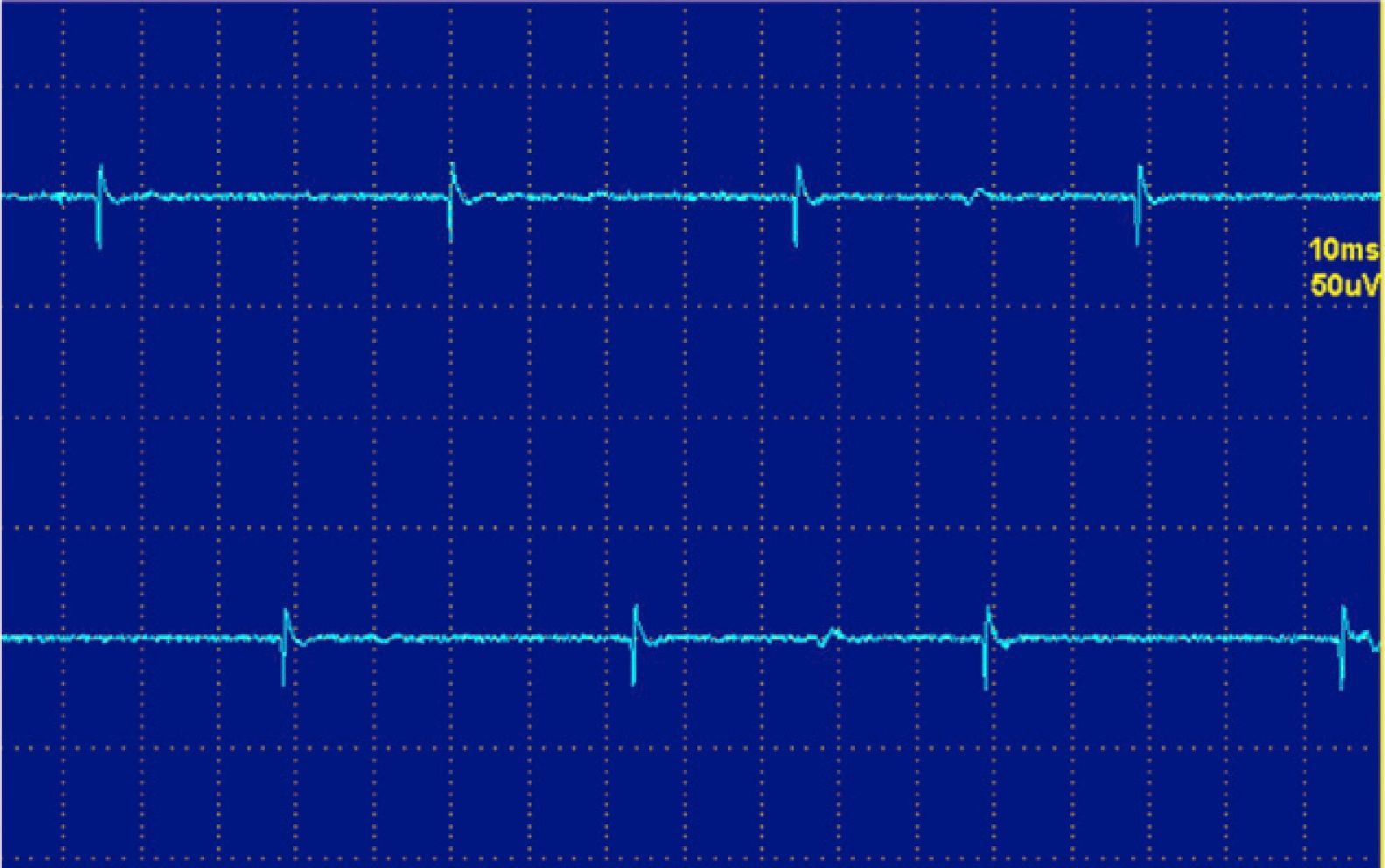
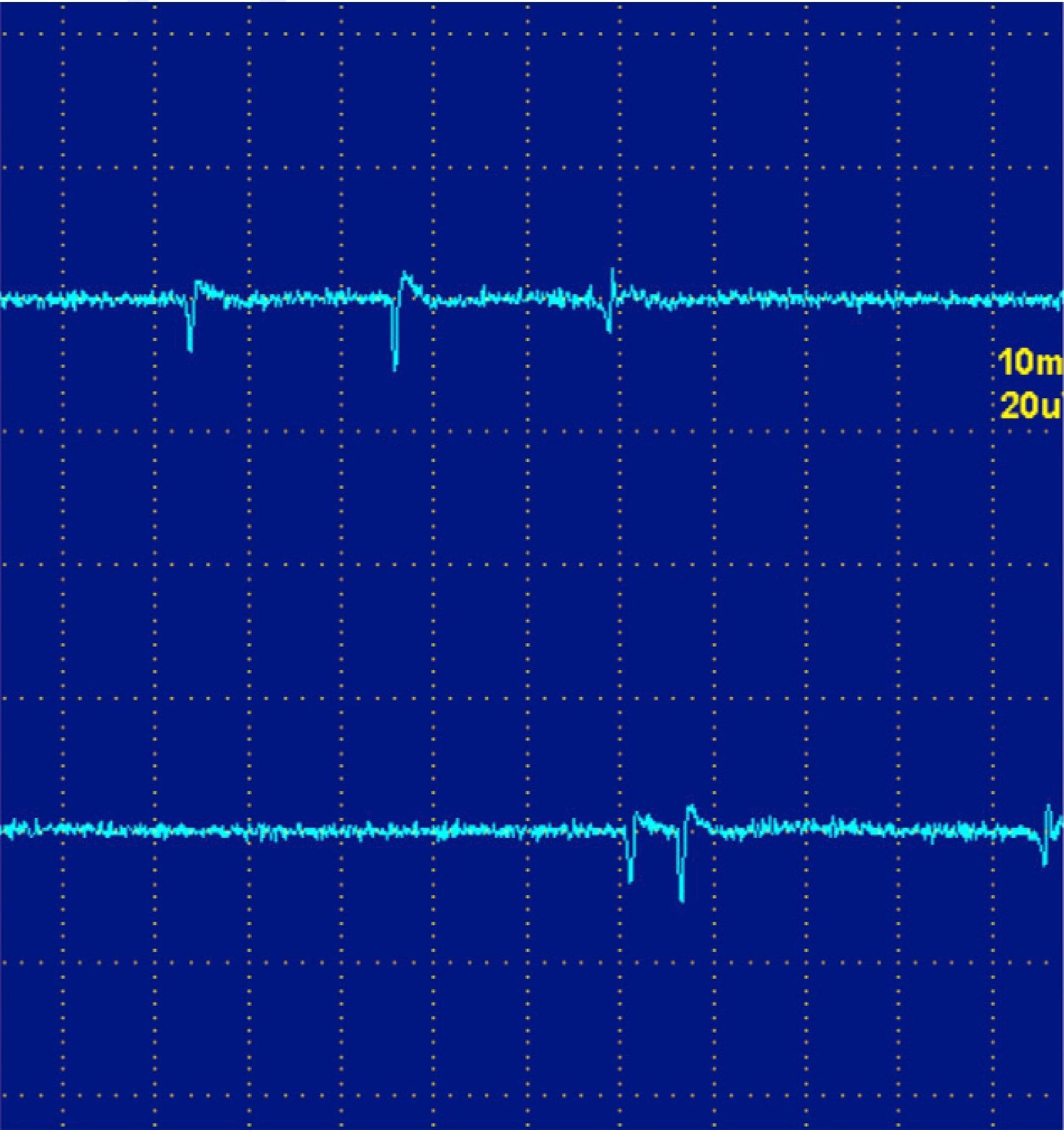
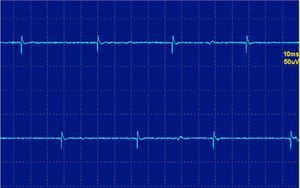
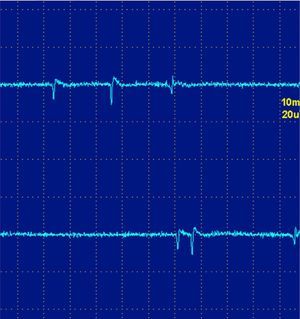
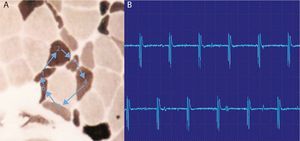
The fibrillations and positive waves (Figs. 1 and 2) are small electric potentials, always positive, always rhythmic, due to the generation of action potentials in isolated denervated muscle fibers.
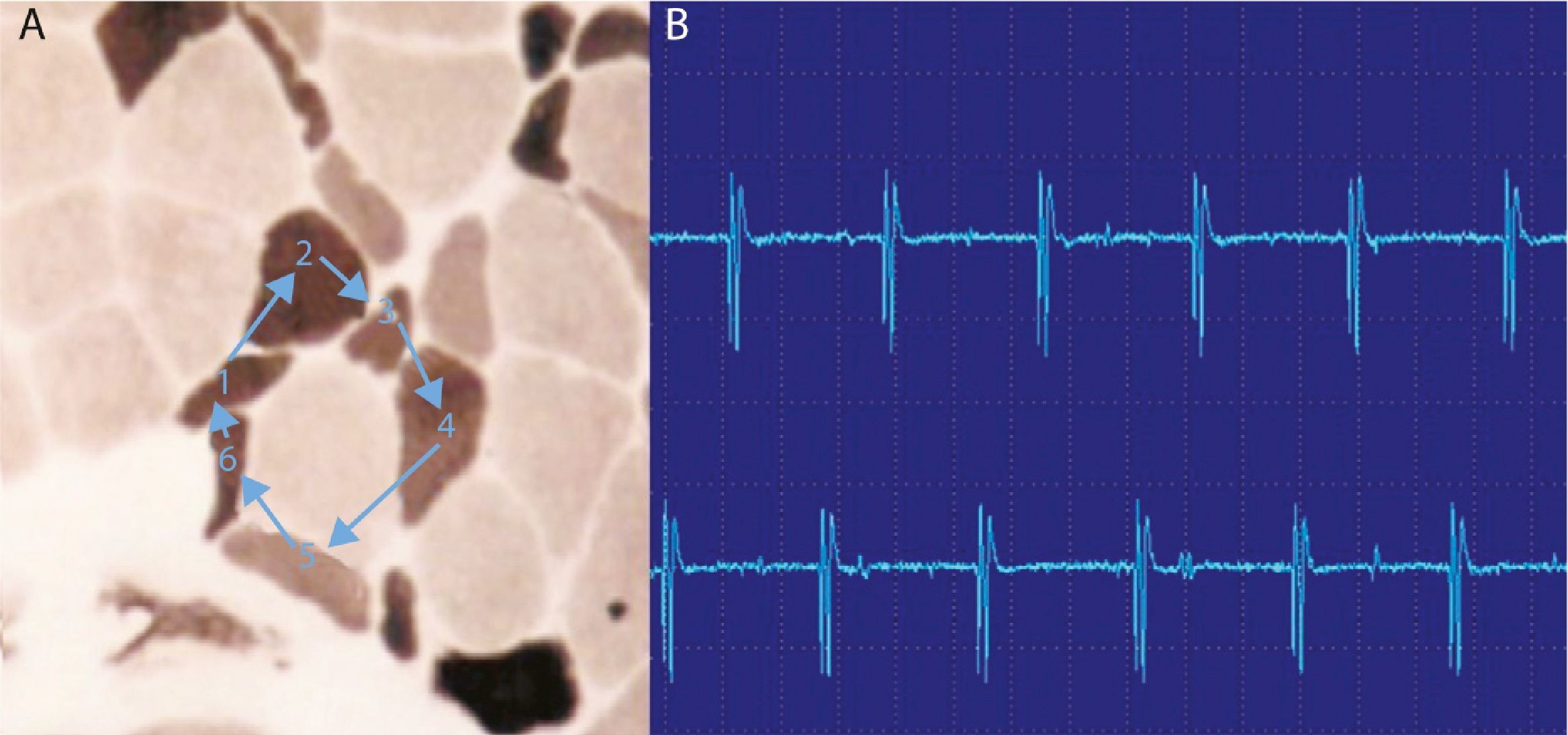
They are typical of denervation, but can also be detected in myopathies with important necrosis or inflammation. It is believed that these phenomena occur in myopathies with muscle fiber necrosis so important that it produces denervation of some fibers. High frequency discharges, also called pseudomyotnic or complex repetitive discharges, have a beginning and an abrupt end and sound like a machine with a rhythmic cadence. Contiguity transmission occurs of a potential generated spontaneously. Processes are also typical of long-standing denervation, and like the fibrillations and positive waves, can be found in IM (Fig. 3).12
High-frequency repetitive discharges. (A) The potential arises spontaneously in muscle fiber number 1 and epfatic transmission, by contiguity, passes to 2, and then to 3–6 and again at 1. (B) The electromyogram polyphasic potential starts and ends abruptly, which is always the same, and fires rhythmically, and is typically a sound like a machine, “Chaca-Chaca-Chaca”.
Myotonic discharges are the electrophysiological representation of the difficulty of relaxation following voluntary or induced muscle contraction, usually after percussion, known as myotonia. They are due to the transient hyperexcitability of the muscle fiber membrane. Myotonia is easily identified clinically by the characteristic difficult and slow muscle relaxation after a contraction maintained for a few seconds. The myotonic phenomenon decreases in intensity after several repeated contractions. From an electrophysiological point of view it reflects the activity of individual muscle fibers that discharge spontaneously and repetitively. It has a distinctive sound like a plane falling sharply, and once identified is easily recognizable. Myotonic discharges are typical of myotonic dystrophy, but can be seen in isolation in IM. They are especially common in antimalarial-associated myopathies.
During voluntary activity the patient is asked to perform mild exertion, in order to observe the isolated motor unit potentials (MUP) and to analyze them properly. It also analyzes the activation and recruitment of muscle fibers, i.e. the way that the muscle has to increase its ability to contract. One will usually ask the patient to make a strong effort with that muscle, usually against resistance.
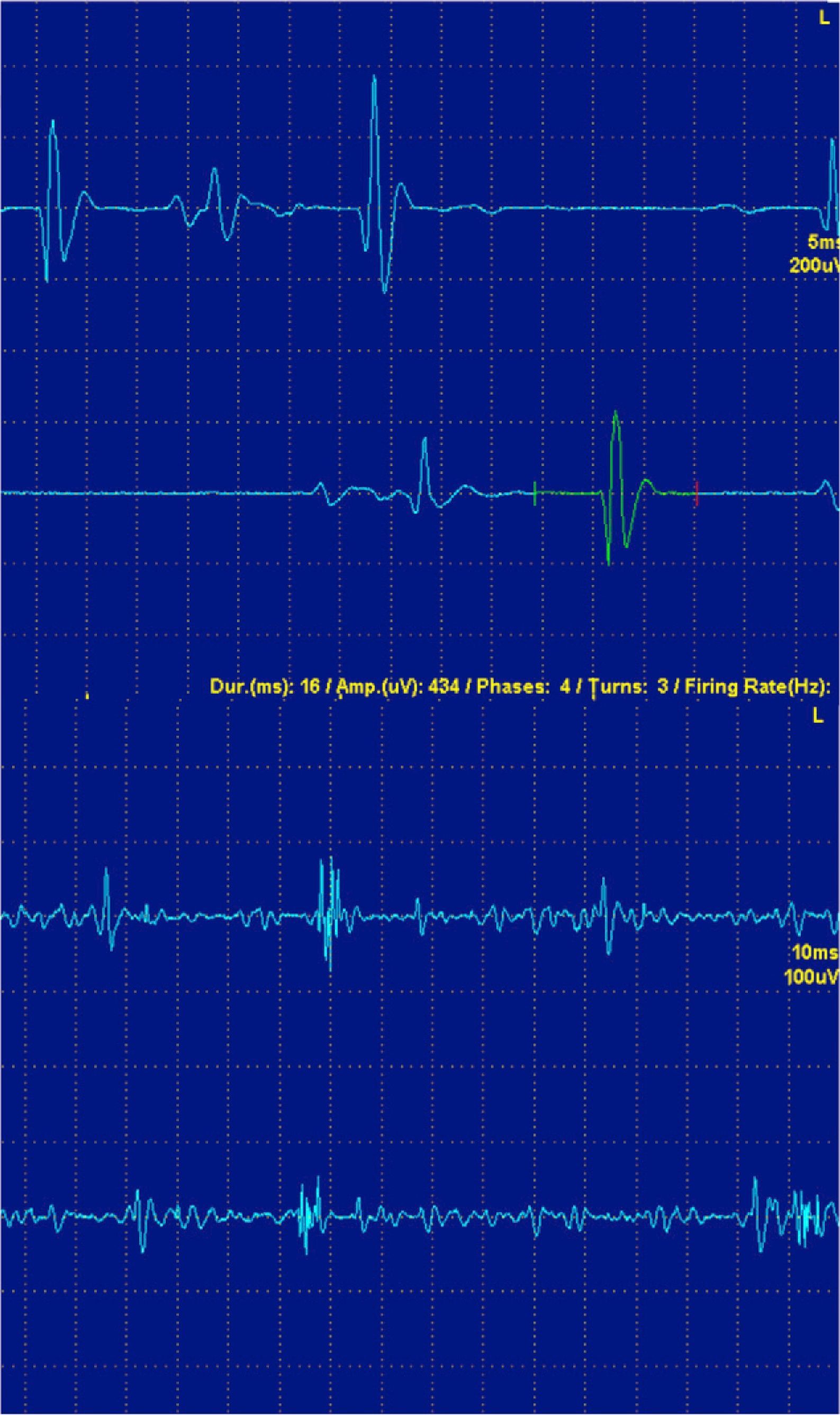
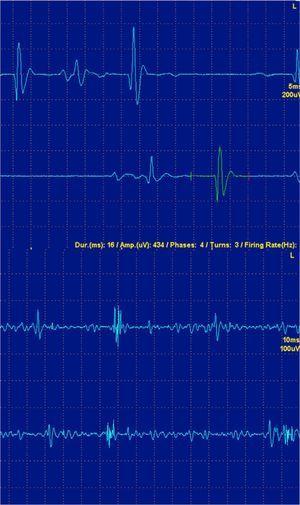
MUP is the summation of action potentials of muscle fibers innervated by a neuron, i.e. reflecting the neurophysiological motor unit. It is recommended that one examines about 20 MUP. We study the amplitude, duration and the number of times that the potential crosses the baseline (directly related to the synchrony of contraction of the fibers), which is called a MUP phase. Those with 5 or more phases are called polyphasic.
In the acute stages of myopathy there is a loss of myofibrils and the synchrony of contraction. For this reason the myopathic MUP are small in amplitude, short duration and polyphasic (Fig. 4).
In the chronic stages of myopathy and particularly in the IM, it is customary for MUP to be larger and of longer duration. These MUP reflect muscle regeneration in units with the greatest number of muscle fibers. In the analysis of recruitment pattern at maximum effort, early recruitment pattern, i.e. a greater number of motor units which are recruiting for a relatively submaximal effort, is usually seen, as the patient has to recruit more motor units for a relatively low strength.13
EMG in IM often shows what is usually defined as a “neurogenic-myopathic pattern” or “mixed pattern”: MUP spontaneous activity with small, short and multi-phase, and early recruitment. This pattern is highly suggestive, but not specific, of an IM. It can also be seen in some myopathies with significant muscle destruction such as facioscapulohumeral dystrophy, some girdle dystrophies, or the dystrophinopathies.
For an EMG it is relatively easy to detect spontaneous activity. It is more difficult to detect myopathic MUP, especially in patients with mild myopathy. Do not forget that the interpretation of EMG is usually subjective and dependent on observer.14
The electromyographic findings in a relatively nonspecific myopathy may be useful, although the presence of a mixed pattern suggests, as mentioned, an IM. Although patterns described for each of the 3 traditional15 disease entities exist, definitive diagnosis is usually made by biopsy.2
Although EMG has been used to monitor response to treatment and the evolution of a myopathy, we, like other authors,16 prefer to use physical examination and CPK levels, especially in polymyositis.
New Techniques: Analysis of Turns-amplitudeThis type of study is based on a mathematical analysis of electromyography, which measures the relationship between the turns and amplitude of the MUP and plots on a graph. This technique allows to differentiate myopathic from neurogenic processes. In the former, this ratio is lower than in the latter. The study is usually done in a pattern of maximum effort.17
Differential DiagnosisIn the study of IM there are different situations that arise in the differential diagnosis with other disease entities and have important implications for diagnosis, prognosis and treatment. Do not forget that the diagnosis of inflammatory myopathy can only be made with certainty using a muscle biopsy.18
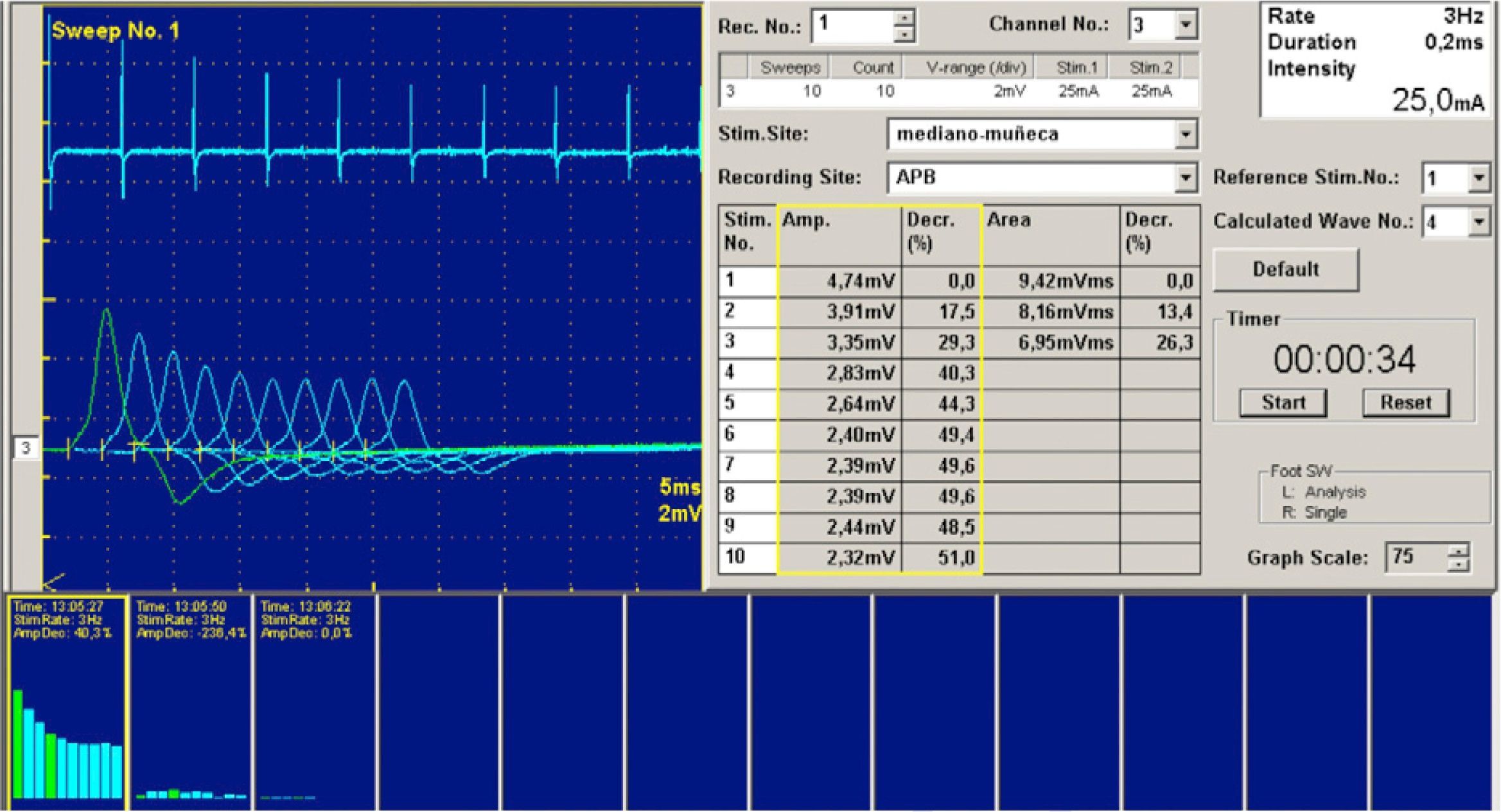
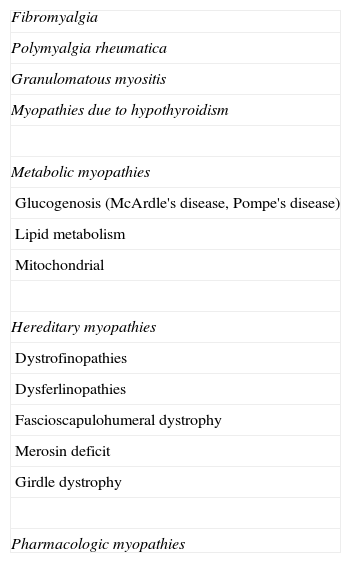
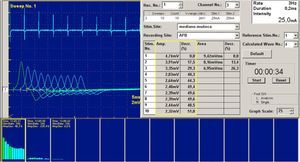
“Pseudopolymyositis” (Table 1): some inherited myopathies such as Duchenne dystrophy or disferlinopathies can occur with acute or subacute CPK elevations, and may lead to misdiagnosis of a IM.19 Some of these diseases have an autosomal recessive inheritance and family history does not necessarily help. The diagnosis can be complicated even with a biopsy, because some of these entities may have inflammatory muscle infiltrates. The EMG does not usually help in these patients because the findings are similar. Some clinical details such as facial weakness or fascioscapulohumeral dystrophy in disferlinopathies with distal involvement may give clues to the diagnosis. The help of an expert in neuromuscular diseases can be of great interest to the rheumatologist. Other myopathies that may present as “pseudopolymyositis” are the metabolic McArdle's disease mitochondrial myopathies, Pompe's disease, parasitic or toxic myopathies, which deserve a separate section. Other simulators of IM are neuropathy or neuromuscular junction diseases such as myasthenia gravis. There is also the possibility that some of these conditions are combined in a single patient, and although this is rare, should be considered when treating IM is not proving effective. As mentioned previously, EMG is not always able to provide data on the differential diagnosis. In myasthenic syndromes such as myasthenia gravis, a congenital myasthenia or Lambert–Eaton syndrome, the EMG, through repetitive stimulation techniques, provides a diagnosis with high specificity (Fig. 5).
False Inflammatory Myopathies.
| Fibromyalgia |
| Polymyalgia rheumatica |
| Granulomatous myositis |
| Myopathies due to hypothyroidism |
| Metabolic myopathies |
| Glucogenosis (McArdle's disease, Pompe's disease) |
| Lipid metabolism |
| Mitochondrial |
| Hereditary myopathies |
| Dystrofinopathies |
| Dysferlinopathies |
| Fascioscapulohumeral dystrophy |
| Merosin deficit |
| Girdle dystrophy |
| Pharmacologic myopathies |
In toxic myopathies, we must remember that not all proximal loss of strength is due to an inflammatory myopathies. There are many other forms of myopathy, drug-induced (Table 2), toxic,20 infectious, hereditary, metabolic, and so on. They may manifest clinically in a manner indistinguishable from an IM. In these diseases the EMG does not help in differentiation, but if the history is carefully collected, the removal of the toxic agent and subsequent clinical improvement usually leads to the diagnosis.
Pharmacologic Myopathies.
| Lipid lowering drugs |
| Statins |
| Ezetimibe |
| Drugs that increase the risk of statin associated myopathies |
| Amiodarone |
| Fibrates |
| Nyacin |
| Cyclosporin |
| Imidazoles |
| Macrolides |
| Verapamil |
| Drugs used commonly by rheumatology |
| d-Penicillamine |
| Colchicine |
| Cloroquine and hydroxicloroquine |
| Interferon alpha |
| Cyclosporin |
| Tacrolimus |
| Steroids |
| Germanium |
Some iatrogenic myopathies that are of particular interest to the rheumatologist are those secondary to drugs used in the treatment of other rheumatologic diseases. One particular case is colchicine21 myopathy. Colchicine is an antimicrotubule drug that binds to tubulin and prevents its polymerization and the formation.22 It produces a myopathy which can be acute and potentially lethal. It can also cause associated neuropathy. As a potent inhibitor of cytochrome P450 CYP3A4, it is able to potentiate the myotoxic action of other drugs such as statins.
In colchicine myopathy, the clinical picture can be superimposed to that of a conventional IM, except that there is often a disproportionate hyporeflexia and strength loss and a mild loss of vibratory sensation, but these are subtle signs upon the exploration and can be easily overlooked. CPK may be slightly high. In this case, the EMG is especially useful, since in addition to a mixed pattern there may be electrical myotonia.23 Furthermore, the EMG is used to study associated neuropathy, which also supports the diagnosis. The treatment consists of removing the colchicine.
Chloroquine and hydroxychloroquine can also produce myopathy.24 In general it is a mild myopathy with CPK elevations and discrete higher LDH, although there have been reports of greater severity.25,26 The EMG usually shows a nonspecific myopathic pattern in 53% of cases.24 In case of suspicion one should withdraw the drug if myopathy is clinically important.
Relapse vs steroid myopathy. A relatively common situation with facing the clinician is the patient complaining of worsening weakness, especially of the proximal lower extremity. When this occurs there is often doubt whether it is a relapse of polymyositis or steroid myopathy. In this scenario, EMG can help, combined with other tests. EMG in relapses often shows signs of active destruction of the muscle, i.e. spontaneous4 activity.27 However, in steroid myopathy, EMG is usually normal. In steroid myopathy, CPK levels also tend to return to normal, or at least improve, and tend to increase in relapses. Occasionally it is necessary to perform a new muscle biopsy to make a diagnosis.
ConclusionsThe diagnosis of IM can be difficult. Along with the clinical findings and laboratory tests, neurophysiological tests allow the clinician to appreciate the richness of the physiology and pathophysiology of the neuromuscular system. Knowing when and what muscles to explore neurophysiologically and interpret the findings of EMG is essential for a correct diagnosis and management of IM.
Conflict of InterestThe authors declare no conflicts of interest.
Please cite this article as: Gutiérrez-Gutiérrez G, et al. Utilidad del electromiograma en el diagnóstico de las miopatías inflamatorias. Reumatol Clin. 2012;8:195–200.