Glucocorticoids, aspirin, antimalarials and conventional immunosuppressants are the mainstay of treatment of Systemic Lupus Erythematosus (SLE). Until recently, the first three were the only agents approved for treatment. A better understanding of the pathophysiology of the immune system has identified new therapeutic targets. In fact, belimumab, a human monoclonal antibody to BLyS inhibitor has become, in recent months, the first drug approved for the treatment of SLE since 1957, underscoring difficulties of all kinds, including economic and organizational ones inherent to clinical trials on this disease. Many other molecules are in various stages of development and soon will have concrete results. In this review, we examined the mechanism of action and most relevant clinical data for these molecules.
Glucocorticoides, aspirina, antipalúdicos e inmunosupresores convencionales constituyen la base del tratamiento del lupus eritematoso sistémico (LES). Hasta recientemente, los 3 primeros eran los únicos agentes aprobados para su tratamiento. El mejor conocimiento de la fisiopatología del sistema inmunitario ha permitido identificar nuevas dianas terapéuticas. De hecho, belimumab, un anticuerpo monoclonal humano inhibidor de BLyS, se ha convertido hace pocos meses en el primer fármaco aprobado para el tratamiento del LES desde 1957, lo que subraya las dificultades de todo tipo, incluyendo las económicas y organizativas, inherentes a los ensayos clínicos sobre esta enfermedad. Otras muchas moléculas se encuentran en distintas fases de desarrollo y en poco tiempo dispondremos de resultados concretos. En esta revisión repasamos el mecanismo de acción y los datos clínicos más relevantes de estas moléculas.
Mortality in Systemic Lupus Erythematosus (SLE) has been significantly reduced in the past 15years but remains high. Bernatsky et al.1 followed 9547 patients for a mean 8.1years and found a global standardized mortality rate (relationship between observed deaths and expected deaths; SMR) of 2.4, higher in the group of patients under 40 (SMR: 10.7) and in those with a time since onset of disease of less than 1year (SMR: 5.4); among the causes of death, the most important is still lupus nephritis (LN) (SMR: 7.9) and infections (SMR: 5.0). In opposition to the severity of these indicators the therapeutic arsenal against this disease up until some months ago was limited to aspirin, hydroxychloroquine and steroids; the rest of the drugs used, including cyclophosphamide, azathioprine, mycophenolate mofetil, cyclosporine A and methotrexate, have not been approved for their use in SLE and have an indiscriminate immunosuppressant effect which often leads to severe adverse events and opportunistic infections. In spite of this, the use of these off label drugs, along with improved management of common comorbidities in this disease, including hypertension, dyslipidemia, nephrotic syndrome, etc., has considerably improved its long-term prognosis, although due to their narrow therapeutic margin leads some patients to present adverse events that worsen their life quality and expectancy, some do not reach an adequate control of the disease and others present frequent flares.2 For these reasons, research into new treatments becomes a universally recognized need. Fortunately, a better understanding of the pathogenic mechanisms involved in it has allowed for the identification of new targets that could improve the efficacy and safety of current treatments,3 although doubts on their cost-effectiveness and long-term safety remain.
A Panoramic View of the Physiopathology of Systemic Lupus ErythematosusIn the pathogenesis of SLE there are at least two well-differentiated compartments. On the one hand, the physiopathology of autoimmunity and its consequence, autoantibodies, which seem a product of interactions genetic and exogenous environmental factors, which act on the immune system leading to events that trigger a flare in a physiologic process, namely autoimmunity. It is known that in this immune response, nuclear antigens and their corresponding autoantibodies lead to an amplifying effect through mechanisms of both innate and acquired immunity. The second compartment of SLE pathogenesis is the development of inflammation and damage in target organs.4
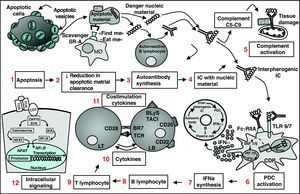
Apoptotic cells seem to be the origin of autoantibodies (Fig. 1); specifically apoptotic vesicles are exposed on the surface of the apoptotic cell, which contains cellular debris, among it present the nucleic acid and nucleotides. Under normal circumstances, it is the monocytic-macrophagic system, mainly macrophages, which is in charge of removing apoptotic debris from the circulation. The complement system participates in this process, through the opsonization of apoptotic particles by C3b.5 Other molecules, secreted by the apoptotic cell, also participate in the process, such as lysophosphatidylcholine, allowing the attraction of phagocytes (find me signals) and molecules exposed on their surface, such as oxidized phosphatidylserine, recognized by scavenger receptors on the surface of the phagocyte, such as CD36 and oxLDL, facilitating their internalization (eat me signals).6 In SLE there are both an increase in apoptosis as well as a deficient clearance of apoptotic cells and both alterations lead to the accumulation of secondary necrotic material which may trigger inflammation, and modified nuclear fragments that are interpreted as danger signals by the immune system, leading to the production of antibodies for their neutralization on the part of self-reactive B lymphocytes.6
Panoramic vision of pathogenesis of Systemic Lupus Erythematosus. The apoptotic cell exposes on its surface apoptotic vesicles with nuclear antigens (1); due to a deficient clearance mechanism by the macrophage system, they accumulate (2) and gain access to autoreactive B lymphocytes, which synthesize autoantibodies (3); the IC formed (4) have the capacity to activate complement and damage tissue (5) and activate PDC (6); these respond by producing IFN-( by TLR7 and TLR9 dependent pathways (7); IFN-α has multiple effects on the immune system that favors autoimmunity, such as the differentiation of B cells to antibody producing plasma cells, T cell activation and dendritic cell maturation. All of these lead to a vicious circle that intensifies and perpetuates the autoimmune process. Other therapeutic targets are BL (8) TL (9), cytokines (10), costimulation molecules (11) and intracellular signaling pathways (12). PDC, plasmocytoid dendritic cells; IC, immune complexes; IFN, interferon; BL, B lymphocyte; MØ, macrophage; TCR, T cell receptor; TLR, toll-like receptor.
This secretion of autoantibodies and their binding to nuclear antigens lead to the formation of immune complexes (IC), fundamentally DNA/anti-DNA, with a capacity to deposit in tissues where, along with the participation of the complement system, will produce lesions, something best exemplified in the glomerurlus.7 These IC with nucleic products are also taken up by plasmocytoid dendritic cells (PDC) through Fcγ-IIAr receptors and engulfed into endosomes. Toll-like receptors (TLR) 7 and 9, anchored in the endosomal membrane, are activated by these IC which sets in motion a transduction cascade leading to the activation of IRF 7/5 and NFκB transcription factors, which derives into the production of great quantities of IFN-α—PDC are the “professional” producers of IFNα—and, to a lesser extent, of other proinflammatory cytokines, such as IL-6.8 The capacity of the IC with nucleic content to unleash an IFN response on the part of PDC is the reason they are known as “interpherogenic complexes”. It is interesting to note that hydroxychloroquine, one of the oldest drugs employed in SLE, which inhibits the binding of nucleic acid strands to these TLRs through the elevation of endosomal pH9 and therefore is considered a TLR inhibiting drug.
IFN-α secreted by PDC has autoimmunity enhancing effects: it promotes the maturation of dendritic cells, activates T lymphocytes (TL) (including autoreactive cells that may have escaped central tolerance) and intervenes in BL differentiation to auto-antibody producing dendritic cells, which in time leads to a larger formation of interpherogenic immune complexes. In this manner, IFN-α is a great activator of the immune system and its appearance completes a circuit (Fig. 1) in which when the presence of plasmatic cells is increased so is autoantibody formation and immune complexes with nucleic acids, which in turn activates more PDC, which then produces more IFN-α and closes a vicious circle that maintains and multiplies the mechanism of autoimmunity.
Microarray studies of oligonucleotides have shown that patients with SLE have an overexpression of INF and granulocyte production-related genes (see below), supporting the hypothesis that IFN-α plays a principal role in the physiopathology of this disease.
Therapeutic TargetsResearch into new targets aims at specific intervention at concrete points of the pathogenic pathways; therefore avoiding conventional immunosuppressant mechanisms that, along with the desirable effect, leads to other undesirable ones. Drugs under current research in SLE and other autoimmune diseases are directed both against cell targets, including BL surface molecules (CD20, CD22, and CD19) and costimulation (CTLA-4, CD40/CD40L, ICOS/B7-H2), as well as extracellular (cytokines, chemokines) and intracellular ones, in other words, molecules that intervene in signal transduction and transcription, such as the proteosome.
Access to many of these targets has been possible thanks to the advent of biotechnological drugs: monoclonal antibodies, and recombinant fusion proteins. Monoclonal antibodies, usually IgG, are identified by the “mab” suffix (as in monoclonal antibody); the suffix “omab” indicates a murine origin; “ximab” refers to chimeric antibodies (approximately 30% of which is murine); “zumab”, to humanized (under 10% murine), and “umab” referring to completely human antibodies. The “cept” suffix is used for recombinant-derived fusion proteins. To these one has to add SMIPs (small modular immunopharmaceuticals), polypeptides that in a single strand integrate the binding, effector and hinge domains, exactly as an immunoglobulin does, but are small and therefore with a better penetration into tissues and cells.10 Intracellular targets, such as tyrosine kinases and Janus kinases, among others, are inhibited through chemical, not biotechnological compounds, with a low molecular weight (<1kDa), called “small molecules”, identified by the “inib” (inhibitor) or “imod” (immunomodulator) suffix. In addition to their size, small molecules have some advantages over biotechnological drugs such as their lower cost, oral administration and the absence of immunogenicity and its consequences, such as the formation of human anti-chimeric antibodies (HACA) or anti-human (HAHA), which inhibit drug action.
ToleragensAs mentioned above, defective clearance of apoptotic debris with nucleic content and a loss of tolerance are considered as the factors that determine the formation of antibodies against nuclear components.11 A target that has called the attention of researchers is to precisely achieve that these nucleic danger signals loose their immunogenic capacity, in other words, to tolerate them with the intention that they not unleash an autoantibody response. With this in mind the so-called “toleragens” have been employed. Among them, abetimus and edretide have come some way but their development was interrupted because, in spite of reducing the production of autoantibodies, do not lead to improvement of disease.
- –
Abetimus (Riquent®, Rentol®). It is a synthetic molecule formed by four deoxynucleotide sequences joined by a polyethyleneglycol base, with the ability of cross linking DNA-specific BL receptors in the absence of T cells and inducing anergy or apoptosis. Tested in several SLE trials, it has been seen to reduce the concentration of anti-DNA antibodies and normalize complement12; however, they do not shorten the onset of a new episode of nephritis, leading to the interruption of the ambitious ASPEN trial, with 890 patients with LN, in 2009.
- –
Edratide (TV-4710). Something similar occurred with edratide, a 19 amino acid synthetic peptide with a sequence based on the complementarity determining region 1 (hCDR1) of an anti-DNA monoclonal antibody,13 whose development was interrupted due to lack of efficacy after the PRELUDE tiral in 2007.
- –
Lupuzor (P140, IPP-201101, Rigerimod). The 140 phosphopeptide formed by phosphorylated residues 131–151 of the 70K protein of the spliceosome inside the U1-RNP antigen has an incompletely understood mechanism of action but seemingly promotes anergy or deletion of self-reactive T lymphocytes, partially or totally antagonizing the T cell receptor (TCR) or through an effect on regulator TL (Treg). A phase IIb trial results show that in addition to being safe and well tolerated, it is capable of reducing the clinical and biological activity of SLE.14 A phase III trial is currently underway looking to confirm these results.
The complement system plays a double role in the physiopathogenesis of SLE: its initial components intervenes in opsonization and clearance of apoptotic bodies so the deficit of the first components lead to the accumulation of dangerous material and predispose the appearance of disease. On the other hand, terminal components intervene in the inflammatory process that leads to tissue damage and is associated with clinical manifestations and disease progression, evidenced by the elevation of serum levels of C5a and C5b–C9 during flares.15 In mouse models, the administration of anti anti-C5 improves the progression of renal disease, delays the appearance of proteinuria and increases survival. For these reasons, inhibition of activation of the terminal sequence of complement, conserving the initial cascade, constitutes an attractive target.
- –
Eculizumab (Soliris®). Humanized monoclonal antibody that blocks C5 and prevents cleavage into proinflammatory fragments C5a and C5b, currently approved for the treatment of paroxystic nocturnal hemoglobinuaria. A phase I trial lasting 56days in SLE patients showed its efficacy and safety and led to improvement in this period of time.15 However, the risk of meningococcal infection represented by the inactivation of the terminal sequence of complement may limit its use in humans. In spite of this, a phase II trial is currently underway to evaluate its safety in membranous lupus nephritis.
- –
Other complement inhibitors. Another monoclonal antibody vs C5, pexelizumab, has been used in acute myocardial infarction and a recombinant antagonist of the human receptor for C5a (C5aRAM; CGS 27913) has been shown to improve nephritis in the mouse MRL/lpr model,16 but for the time being neither has been used in humans.
Dendritic cells are a heterogeneous group of cells derived from the bone marrow, which participate in immunovigilance, antigen presentation and tolerance. There are 2 subtypes, conventional dendritic cells and PDC, the latter playing a principal role in the pathogenesis of SLE.8 PDC participate in innate immunity against virus and intracellular bacteria through TLR7 (for single stranded RNA) And TLR9 (for hypomethylated DNA), localized in the endosome and whose activation results in the secretion of type I interferons and among them, IFN-α.17 The great quantities of IFN-α found in patients with SLE, disproportionate to the low concentration of PDC in peripheral blood, are attributed to the presence of activated PDC in lymphoid nodules, skin and other target organs.8
- –
IRS 954. The potential role of PDC as a therapeutic target has been tested indirectly in SLE through inhibition of TLR, intending to stop intracellular signaling leading to synthesis of IFN-α by these cells and avoiding its production. Oligo-deoxynucleotides (ODN) containing non-methylated CpG sequences act as antagonists of TLR9, which has led to the development of modified sequences substituting them for methylated ribonucleotides, obtaining antagonists for TLR9, TLR7 or both.18 One of those dual antagonists, IRS 954 has been tested in a mouse model (NZB×NZW)F1, showing an increase in survival and a reduction in specific autoantibody levels, as well as in proteinuria, severity of glomerulonephritis and organ damage.19
- –
ST2825. The initial signal produced by TLR7 and TLR9 is transduced exclusively through the adaptor protein MyD88, whose absence reduces renal damage in mouse models.20 A mimetic peptide called ST2825, which interferes with MyD88, interrupting the signaling pathway of these TLRs. ST2825 has shown in vitro to block the activation induced by TLR9 in self-reactive memory BL obtained from SLE patients.21 In spite of this being a promising treatment, it has still not been tested in human SLE.
Current experimental and genetic evidence indicates that IFN-α is a key mediator in the pathogenesis of SLE. In addition to its elevated levels on the blood of patients with SLE and its known effect on BL and TL, studies with oligonucleotide microarrays showing that up to 70% of adults and 95% of children with SLE have an overexpression of IFN and granulocyte production related genes have to be added, introducing the notion that neutrophils play a pathogenic role in SLE. This pattern of gene expression is called the IFN signature.22 This signature has been found in other autoimmune diseases, such as RA and multiple sclerosis (MS) and not all patients with SLE have this characteristic, making it more a signature of autoimmunity than of SLE. A process called necrosis extracellular trap (NET), occurring after the death of neutrophils (therefore called NETosis), could be the link between IFN and neutrophils in the pathogenesis of SLE.23 Both for its biologic and genetic effects, IFN-α is considered one of the most sought after therapeutic targets today. 2 monoclonal antibodies block IFN-α, sifalimumab and rontalizumab, and immunization against this cytokine through a cuinoid has started being tested in humans.
- –
Sifalimumab (MEDI-545). A completely human anti-IFN-α antibody, it has been tested in phase I trials showing a dose dependent inhibition of the expression of IFN-α induced genes (IFN signature), as well as a tendency to improve activity with respect to the placebo group, a reduced need for immunosuppressants and a significant reduction in flares.24 A phase IIb trial is currently in its recruitment stage.
- –
Rontalizumab. An anti-IFN-α humanized antibody currently undergoing phase II trial (ROSE) in 210 patients with moderate to severe SLE (active July 2011).25
- –
IFN-α-quinoide. An alternative to the administration of monoclonal antibodies is the generation of a natural secretion of anti-IFN-α neutralizing antibodies, akin to a vaccine, called quinoid. Quinoids are being developed vs TNF-α and al VEGF. Quinoids are formed by heterocomplexes of the target cytokine, in this case IFN-α, with a carrier protein such as barnacle hemocyanin, which induce a natural polyclonal response vs IFNα epitopes. A mouse model, where this IFN-α-quinoid was used induced neutralizing antibodies without a cell response or apparent collateral effects, capable of controlling lupus manifestations, including renal lesion.26 The results of a phase I-II in patients with mild to moderate SLE have recently been presented27 and indicate that IFN-α-quinoid leads to a strong enough anti-IFN-α antibody response to neutralize the IFN signature and elevate C3 levels.
Along with IFN-α’s important role, IFN-γ intervenes in the pathogenesis of SLE by being a potent mediator of inflammation and inducing the expression of the BL soluble stimulator (BLyS).28 A completely human monoclonal antibody has been developed against IFN-γ (AMG 811, fontolizumab), with phase I trials in recruitment stages for LN29 and discoid lupus.30
B Cell DepletionAnother point where the pathogenic circle that we have referred to can be inhibited (Fig. 1) is BL, aiming for their depletion.31 Although the initial objective was to reduce the production of auto-antibodies it has been noticed that B cells, in addition to synthesizing antibodies, have other equally important functions: they are efficient antigen presenting cells (APC), regulate the function of self-reactive T cells, influence other APC and produce diverse cytokines. Regulator B cells (Breg) constitute a recently described subpopulation that produce IL-10 and TGFβ, 2 cytokines with immune suppressing activity: they inhibit T cell differentiation to an inflammatory Th2 phenotype as well as activation of macrophages and the proinflammatory functions of dendritic cells, among others.32 These BL regulating functions are induced through activation of the B cell receptor (BCR, CD40) or TLR9 and mediated by IL-10. This explains why B cell depletion not only leads to a diminished production of antibodies but also produces many more effects, some of which are beneficial in suppressing autoimmunity and therefore convenient to maintain. The concept of Breg cells will oblige researchers, sooner or later, to reanalyze B cell depletion therapeutic strategies.
B cell depletion can be achieved by 2 pathways: through monoclonal antibodies vs surface molecules, such as CD19, CD20, and CD22, or inhibiting survival factors such as BLyS (B lymphocyte stimulator) or APRIL (A proliferation-inducing ligand).
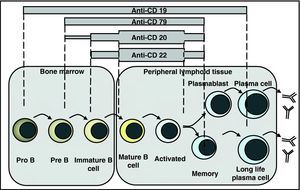
Anti-CD20 AntibodiesCD20 is a membrane protein expressed on pre B and mature B lymphocytes, but not plasma cells (Fig. 2). In light of the central roles BL play in the pathogenesis of SLE, a lot of research has delved onto the development of at least 6 anti-CD20 monoclonal antibodies, each with its own characteristics. In addition to rituximab (RTX), there is ocrelizumab (OCZ), with a greater affinity for CD20 than its competitors; ofatumumab that induces greater apoptosis and has been tested in MS; veltuzumab, tested only in lymphoma, and finally two drugs produced with SMIP technology: SBI-087 and TRU-015. All of these lead to B cell depletion through a triple mechanism that to a certain degree involves apoptosis, cell mediated cytotoxicity or antibody-mediated cytotoxicity.
- –
Rituximab. 2 large randomized clinical trials with RTX in SLE have been undertaken. The EXPLORER trial, in 169 patients with non-renal and non-neurological SLE, with moderate to severe activity, had the objective of evaluating complete or partial response based on the BILAG scale and the absence of flares. No difference with placebo was found; however, the response in Hispanics and African Americans was better (33.8% vs 15.7%; P=.04) and an important reduction in anti-DNA levels as well as improvement of hypocomplementemia was seen.33 The LUNAR trial, done in 72 patients with type III and IV LN had the objective of evaluating complete and partial responses at week 52 of treatment. In spite of a larger number of responders in the RTX group, no significant difference was found compared to placebo. As in EXPLORER, better responses and anti-DNA normalization was seen in African Americans and hispanics.34 Both studies used 2 doses of 1g separated by 15days.
Spectrum of B cell depleting therapies: anti-CD20, anti-CD22 and anti-CD79 antibodies have an overlapping spectrum of action, including pre B or immature B cells up to activated and memory B cells, without affecting plasma cells. The anti-CD19 spectrum extends from pro B to plasmablasts and some plasma cells.
These results stand in contrast with several open studies that found improvement with RTX in approximately 60% of cases and a renal response in up to 66%.35 This has led to controversy and a detailed analysis of the methodology employed. Among the possibilities explaining these discrepancies are the fact that some trials allowed the use of steroids and immunosuppressants that could have masked the antiCD20 effect that studies were too short (with RTX being more effective in the medium to long terms) that they included patients who were not severely ill and that BILAG is a very demanding score to evaluate response to treatment.
- –
Ocrelizumab. Humanized anti-CD20 that theoretically reduces the development of HACA that occur with the chimerical RTX. It has been tested in the BELONG trial in patients with type III and IV LN, in which 127 patients received the 400mg dose and 128 received 1000mg. The objective was to evaluate the both complete and partial responses at weeks 48 and 96; however, the study had to be interrupted due to an unexpected incidence of severe adverse events, including severe infections. The analysis of the results obtained up until its untimely interruption. At week 32, showed a numerical superiority in the OCZ group compared to placebo, but this difference was not statistically significant, and no differences were found between doses.36
The BEGIN trial, a phase II study designed to evaluate safety and efficacy of OCZ in patients with moderate to severe SLE without renal affection was interrupted due to negative data attained by RTX in a study with overlapping designs.
- –
SBI-087. Is a SMIP10 developed for SLE and currently undergoing phase I studies and with preliminary results that suggest it is well tolerated.
- –
TRU-015. It is also a single chain against CD20 with a longer half life than conventional anti-CD20, designed to increase the potency of antibodies acting through cellular cytotoxicity; its phase II results in RA are promising and confirmed its capacity to produce B cell deletion. Phase I studies have been done in SLE.
CD22 is a carbohydrate binding transmembrane glycoprotein, like sialic acid, with an Ig domain on its N-terminal. Once active it is internalized and functions as a receptor-inhibitor regulating migration and BL activity.2
- –
Epratuzumab. EPZ is the only anti-CD22 used in SLE. The first 2 trials, ALLEVIATE A (moderate SLE) and ALLEVIATE B (severe SLE), evaluated the efficacy of 360mg/m2 and 720mg/m2 doses of EPZ vs placebo, but were suspended due to lack of drug availability. In spite of this, result analysis at the moment of its final precipitate demonstrated an important reduction in clinical activity and steroid requirements, as well as improvements in the quality of life.37 The third trial, EMBLEM, was carried out in 227 patients with moderate or severe SLE excluding LN III–IV and severe neuropsychiatric lupus; it showed a significant difference in the percentage of responding patients in the active group compared to placebo (43% vs 21%; P=.02), with no differences in adverse events.38 These promising results have led to a global trial, EMBODY, with patient recruitment currently underway.
Although no trials exist for human lupus, other potential surface molecules that may act as therapeutic targets on B cells are:
- –
Anti-CD79. CD79 is a BCR associated transmembrane protein with an overlapping spectrum of depletion with anti-CD20 (pre-B to mature B) (Fig. 2 but acts through a combination of depletion and BCR signal interruption.
- –
Anti-CD19 (MDX-1342). Probably the likeliest candidate for an SLE trial, it has a depletion spectrum ranging from pro-B to a fraction of plasma cells31 (Fig. 2). So far it has only been tested for RA and hematologic diseases.
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of data. The authors declare that no patient data appears in this article.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.
Conflict of InterestAntonio C. Zea Mendoza is PI for clinical trials by Abbott Lab., MSD, Astra-Zeneca and UCB Pharma.
Alina L. Boteanu collaborates in a study with Astra-Zeneca.
Walter A. Sifuentes Giraldo, María J. García Villanueva and Ana Lois Iglesias have no disclosures to make.
Please cite this article as: Sifuentes Giraldo WA, et al. Nuevas dianas terapéuticas en el lupus sistémico (parte 1/2). Reumatol Clin. 2012;8:201–7.