Although hypersensitivity pneumonitis is the most common pulmonary complication described during treatment with methotrexate, other complications like lymphoproliferative and infectious disease may be considered in the study of respiratory disease associated to methotrexate. The existence of an increased risk to developing infectious diseases may be similar to that observed during treatment with antagonists of tumor necrosis factor and corticosteroids, where Cytomegalovirus pneumonia is a serious complication; early diagnosis and treatment will prevent a potentially fatal outcome.
Si bien la neumonitis por hipersensibilidad es la complicación pulmonar más frecuentemente descrita durante el tratamiento con metotrexato, existen otras complicaciones de tipo linfoproliferativo e infeccioso que hay que tener en cuenta dentro del diagnóstico diferencial ante una neumopatía en el contexto de dicho tratamiento. La existencia de un riesgo incrementado de desarrollar procesos infecciosos o reactivaciones de microorganismos latentes puede ser similar al observado a lo largo del tratamiento con antagonistas del factor de necrosis tumoral y corticoides. Dentro de ellas, la neumonía por citomegalovirus es una complicación muy severa a tener en cuenta, ya que el diagnóstico acertado y el tratamiento oportuno evitarán un desenlace potencialmente mortal.
Pulmonary complications that may develop in patients treated with methotrexate are classified as inflammatory, infectious and lymphoproliferative.1,2 Of these, hypersensitivity pneumonitis is the most common, severe and unpredictable complication, with an incidence of 1%–3% and which currently, with the advent of more sophisticated diagnostic methods, can reach up to 7% as reported in some recent studies, with a mortality of up to almost 25%.3 Lymphoproliferative complications, mainly non-Hodgkin's lymphomas, can occur during treatment and even after stopping it and are a direct result of immunosuppression and tumor cell proliferation.4 Finally, given the immunomodulatory effect of methotrexate, the risk of developing an infectious process is increased all along the treatment, with the most frequently described being Pneumocystis jiroveci, cytomegalovirus (CMV), varicella zoster, Nocardia and mycobacteria, among others, of whom P. jiroveci accounts for almost 40% infectious complications in some series.2,5 The clinical presentation is often nonspecific and common to all, and may have its onset with progressive dyspnea, cough, fever, tachypnea, hypoxemia and dry rales, featuring, on the chest radiograph, a diffuse interstitial and/or reticular infiltrate, predominantly in lower lobes.6
Here, we present a case of subacute severe lung disease in a patient with polymyalgia rheumatica under chronic treatment with methotrexate, where the approach of an appropriate differential diagnosis and subsequent timely treatment allowed a favorable clinical outcome.
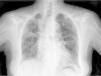
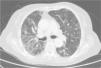
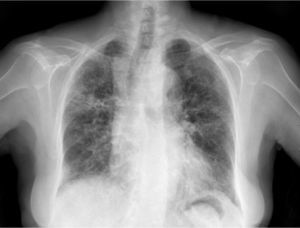
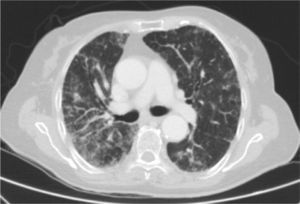
Clinical CaseThe patient is an 83-year-old woman with a history of hypertension treated with amlodipine 5mg/day, chronic kidney disease stage III, secondary to nephrosclerosis (GFR 45ml/min) and chronic treatment of polymyalgia rheumatica with methotrexate 15mg/week, for 4 years approximately. In recent years she has shown good clinical and analytical stability, not requiring steroid therapy or adjustments to her regular medication. Six weeks before admission she progressively presented fatigue, dyspnea, cough, and weight loss of 5kg. She had no fever, joint pain or muscle weakness. On admission, dyspnea at rest was observed with a baseline oxygen saturation of 90%, pulmonary auscultation showed bilateral fine crackles, with no palpable lymphadenopathy or organ enlargement, elevated erythrocyte sedimentation rate of 69mm C-reactive protein 3.5mg/dl and a chest X-ray which highlighted a diffuse and asymmetric bilateral reticular infiltrate with peripheral involvement, worse on the bases and on the right upper lobe (Fig. 1). After making a differential diagnosis of subacute lung disease in the context of methotrexate use, our main approach was to discontinue treatment, start steroids intravenously 48mg/day of methylprednisolone, and support measures, without clear improvement. A high resolution chest CT scan (HRCT) was performed, showing patched interstitial thickening of peripheral predominance in the lung bases and small nonspecific mediastinal lymphadenopathy (Fig. 2). The pulmonary function tests showed forced spirometry values in the low limit of normal, with a forced vital capacity (FVC) 1340ml (82%), a volume of expiratory flow after one second (FEV1) of 1130ml (88%) and an FEV1/FVC ratio of 84%, associated with a severe decrease in lung diffusion of carbon monoxide (DLCO) of 33% and corrected for an alveolar volume (KCO) of 54%. In the rest of the complementary tests, no changes were seen in the blood count, serology for atypical pathogens, the study of tumor markers, blood cultures, the serial study of sputum or the Mantoux/Booster test, so a bronchoscopy was performed with bronchoalveolar lavage (BAL) and led to a positive result in polymerase chain reaction (PCR) testing for CMV associated with an increased rate of CD4/CD8 lymphocytes 3.47, with the rest of the study for common germs, tuberculosis, P. jiroveci and fungi being negative. The microbiological study detected IgG (+), IgM (−) for CMV and CMV viral load in plasma (+), so treatment was initiated intravenously, adjusted for renal function, with ganciclovir 300mg/12h for 14 days, then changing to valganciclovir 900mg/12h orally for 14 days, with good tolerance and clinical response, with improvement in radiological, serological (CMV viral load undetectable) and respiratory function parameters (57.52% DLCO, KCO 80.90%, FVC 1950ml [119%] and FEV1 of 1680ml [130%]).
Although hypersensitivity pneumonitis secondary to methotrexate treatment is a pulmonary complication of this drug2,3,6 described in the literature; some series, as that by Sathi et al., mention that its frequency would be lower if it was diagnosed based on clinical, radiographic abnormalities, thoracic HRCT results, findings in BAL, histopathology and therapeutic response after stopping the medication, so that in their assessment and differential diagnosis other entities should be considered, including infectious processes, pulmonary fibrosis, infiltrative processes, etc.7 Regarding the association between development of infectious processes and methotrexate, it has been demonstrated that there is an increased risk of developing infections due to common microorganisms, larger than that with any other disease modifying antirheumatic nonbiological drug (DMARD); however, its role has not yet been clearly established in the risk of infections or reactivations by opportunistic pathogens, such as those observed during treatment with antagonists of tumor necrosis factor and corticosteroids (prednisone >10mg/day or equivalent).8,9
CMV is a DNA virus belonging to the herpesvirus family, which is spread by direct contact with body fluids, sexual contact, transfusion or transplant; CMV infection in the immunocompetent population usually is asymptomatic, remaining latent, with a seropositivity rate in the general population ranging from 30% to 70%, but which can occasionally cause severe complications with significant morbidity and mortality.10 The reactivation of this infection can occur throughout life, increasing in risk when some type of immunosuppression occurs secondary to another disease, infections or treatments.11 The development of CMV pneumonia is a serious complication that is characterized by progressive respiratory failure and diffuse interstitial infiltrate on the chest X-ray, making early diagnosis and treatment with antiviral drugs such as ganciclovir and/or valganciclovir determinant.10,12 Diagnosis is based on elevated IgM or IgG to CMV, a radiological study that supports the diagnosis, a positive PCR for CMV in the BAL and histological study, which reveal the presence of inclusion bodies (the use of PCR for CMV in blood of immunocompetent patients is usually negative, but if positive, is diagnostic).13,14
ConclusionsDue to all of the above, when addressing the management of interstitial lung disease in a patient on chronic treatment with methotrexate, although hypersensitivity pneumonitis is the usually, more published complication, one should consider other diagnostic possibilities, such as an infectious etiology, because the treatment is completely different and could even overshadow the prognosis. Furthermore, although there is controversy regarding the development of opportunistic infections, this case and others described in the literature reinforce the idea of expanding our range of possibilities, through which a correct diagnosis and treatment of a potentially fatal complication will be achieved.
Ethical ResponsibilitiesProtection of human and animal subjectsThe authors declare that no experiments have been performed on humans or animals.
Confidentiality of dataThe authors state that patient data does not appear in this article.
Right to privacy and informed consentThe authors state that patient data does not appear in this article.
Conflict of InterestThe authors have no conflicts of interest.
Please cite this article as: Ramírez Huaranga MA, Bencosme de Mendez E, Cuadra Díaz JL, Lázaro Polo J, Bujalance Cabrera C. Neumonitis por citomegalovirus durante el tratamiento crónico con metotrexato. Reumatol Clin. 2014;10:328–330.